

Biomass accumulation and partitioning of tomato under protected cultivation in the humid tropics
Kleinhenz, V.; Katroschan, K.; Schütt, F.; Stützel, H., 2006
European Journal of Horticultural Science, 71, 173-182
Europ.J.Hort.Sci., 71 (4). S. 173–182, 2006, ISSN 1611-4426. © Verlag Eugen Ulmer KG, Stuttgart
Biomass Accumulation and Partitioning of Tomato under Protected Cultivation in the Humid Tropics
V. Kleinhenz1), K. Katroschan1), F. Schütt1) and H. Stützel2)
(1) Asian Institute of Technology, ASE/SERD, Klong Luang, Thailand and 2) Hanover University, Faculty of Horticulture, Institute of Vegetable Production, Hanover, Germany)
Summary
Results of a 2-year structural analysis of indeterminate tomato (Lycopersicon esculentum Mill.) cultivated during different seasons under protected cultivation (ventilated greenhouses with PE-film roofs and PE-net walls) in the humid tropics of Central Thailand are presented. Under the prevailing high-radiation conditions (ø 35.9 MJ m-2 outside and 23.7 MJ m-2 inside of the greenhouse), total dry-mass production was 2.6 g MJ-1 and similar to regions at greater latitude with much lower global radiation. Plant density (2.1 plants m-2 in single rows vs. 4.2 plants m-2 in double rows) had no meaningful effect on biomass production and partitioning on a per-plant basis as well as on internode length and specific leaf area (SLA) indicating that availability of light had only limited effect on growth in closer stands. Although crop growth rate (CGR) was comparable to other studies (3-14 g dry mass m-2 day-1), biomass partitioning into individual plant organs was not. The most striking difference to greenhouse tomato production at greater latitude was the low percentage (16-19 %) of total biomass distributed to fruits. Crop responses to lack of sink strength resulting from poor fruit set were deformed leaves and accelerated growth of auxiliary shoots. When canopy density was increased by cultivating tomato with double stems, total fruit biomass per plant was significantly improved by ca. 13 %. Within these plants, ca. 100 % more biomass was partitioned into fruits of the primary stem than the secondary stem. Since leaf biomass and area did not vary significantly between individual stems, there was indication that secondary stems improved availability of assimilates which promoted biomass partitioning into fruits on primary stems. Besides marginally decreasing greenhouse air temperature through greater transpiration, high plant and stem density maximize assimilation and are, therefore, one measure to improve tomato fruit biomass under hot tropical conditions. The generally low amount of fruit biomass was also due to lack of pollination and, therefore, development of parthenocarpic fruits under high temperatures particularly during night. A practice to improve the latter includes application of growth regulators to improve enlargement of parthenocarpic fruits.
Keywords. biomass - growth - partitioning - tomato - tropic - greenhouse
Zusammenfassung
Trockenmassebildung und -verteilung von Tomaten in geschütztem Anbau in den feuchten Tropen
Die zweijährige Untersuchung wurde mit indeterminierten Tomaten während verschiedener Jahreszeiten unter geschütztem Anbau (Netzhäuser mit Folienbedachung) in den feuchten Tropen Zentralthailands durchgeführt. Unter den vorherrschenden klimatischen Bedingungen mit hoher Strahlungsintensität (ø 35,9 MJ m-2 außerhalb und 23,7 MJ m-2 innerhalb der Netzhäuser) war die Gesamttrockenmasseproduktion mit 2,6 g MJ-1 ähnlich der in gemäßigten Breiten mit weit niedrigerer Globalstrahlung. Die Anordnung von Tomaten in Einzel- oder Doppelreihen (2,1 Pflanzen m-2 in Einzelreihen und 4,2 Pflanzen m-2 in Doppelreihen) hatte keinen Einfluss auf Trockenmasseakkumulierung und –verteilung sowie Internodienlänge und spezifischer Blattfläche, so dass anzunehmen ist, dass Verfügbarkeit von Strahlung kein wachstumsbegrenzender Faktor in dichten Beständen war. Obwohl die Bestandeswachstumsrate von 3-14 g Trockenmasse m-2 Tag-1 denen in anderen Studien veröffentlichten Raten entsprach, war die Verteilung von Biomasse innerhalb der Pflanze sehr unterschiedlich: Den größten Unterschied machte der geringe Prozentsatz der Fruchttrockenmasse (16-19 %) an der Gesamttrockenmasse. Der durch geringen Fruchtansatz entstandene Mangel an Sink-Stärke manifestierte sich in eingerollten Blättern und übermäßigem Wachstum von Seitentrieben. Wurde die Triebdichte durch Kultivierung von Pflanzen mit zwei Trieben erhöht, wurde die Gesamtfruchtbiomasse pro Pflanze um 13 % deutlich verbessert. Innerhalb dieser Pflanzen wurde ca. 100 % mehr Trockenmasse in die Früchte des primären Triebs als des sekundären Triebs verteilt. Da es kaum Unterschiede in Blattmasse und -fläche zwischen den einzelnen Trieben gab, kann angenommen werden, dass der zweite Trieb in erster Linie als zusätzliche Quelle von Assimilaten dient, die ungehindert zu den Früchten des primären Triebs transportiert werden können. Neben der geringfügigen Kühlung über Erhöhung der Transpiration ist daher hohe Pflanzen- und Triebdichte zur Maximierung von Assimilation ein geeignetes Mittel um Fruchtbiomasse von Tomaten in tropischen Gewächshäusern zu verbessern. Die durchweg geringe Fruchtbiomasse beruhte auch auf mangelhafter Bestäubung und daraus resultierender Entwicklung parthenokarper Früchte unter Hochtemperaturbedingungen besonders während der Nacht. Diesem Problem kann durch den Einsatz von Wachstumsregulatoren zur Vergrößerung parthenokarper Früchte entgegengewirkt werden.
Introduction
Crop growth models can be of multiple use in research as well as commercial crop production. For development of such models, it is necessary to have detailed knowledge on crop growth and development incorporating the underlying processes such as biomass accumulation and partitioning beyond the level of yield (De Koning 1989). Although tomato is one of the most important vegetable crops worldwide, detailed studies about the dynamics of tomato growth and development are scarce and limited to greenhouse conditions under temperate conditions (e.g. Jones et al. 1991, Dayan 1993). Most research on production of tomato in warm climates has focused on genetic improvement for developing heat tolerance and disease resistance (e.g. Opeña et al. 1993), and crop management for alleviating physiological stress (Kleinhenz 1997) under field conditions. To our knowledge, there is no study about biomass accumulation and partitioning of indeterminate tomato under protected cultivation in the humid tropics.
De Koning (1989) and particularly Heuvelink (1995a) have presented detailed information on accumulation and partitioning of biomass in indeterminate tomatoes cultivated under greenhouse conditions in Northern Europe. De Koning (1989) studied growth under commercial conditions of an entire cropping season of eleven months whereas Heuvelink (1995a) summarized data of twelve growth experiments conducted for a period of ca. three months each.
Several authors have covered the effects of plant or shoot density on tomato yield parameters. Higher density usually resulted in greater total yield and number of fruits but smaller fruit size (e.g. Stoffella et al. 1988, Saglam et al. 1995). This was attributed to greater leaf area indexes (LAI) and, therefore, light interception under narrow plant spacing (Papadopoulos and Ormrod 1991, Papadopoulos and Pararajasingham 1997). Papadopoulos and Ormrod (1988a), and Papadopoulos and Ormrod (1988b) have explained these effects by differences in light penetration and photosynthetic rate at different levels within the tomato canopy. Not for tomato but for indeterminate Vicia faba, Stützel and Aufhammer (1991) have described the favorable effects of isometric plant arrangement on light interception and biomass production.
Precise information on tomato biomass accumulation and partitioning to different plant parts with reference to climatic data is particularly limited. Heuvelink (1989, 1995b) has investigated the effects of temperature on growth and biomass allocation of tomato but only under levels too low to be representative for tropical conditions.
The scope of this paper is to provide information about the dynamics of tomato growth under humid tropical conditions. A structural analysis of tomato cultivated for 19 weeks during different seasons under protected cultivation in Central Thailand is presented. Effects of different plant and stem densities on time courses of biomass accumulation are quantified and modeled with regression analyses. Differences in biomass allocation to different plant parts are discussed against the background of varying climatic conditions and compared with published results from similar growth analyses in Northern Europe.
Materials and Methods
Study site
Trials were located at a newly established 0.28-ha greenhouse area at the Asian Institute of Technology (AIT; 14° 4’ N, 100° 32’ E), 42 kilometers north of Bangkok in the tropical lowlands of the Central Region of Thailand. Climatic conditions comprise three seasons: the cooler-dry season from November to February, the hot-dry season from March to May and the hot-wet season from June to October with mean temperatures of 26.5, 29.6 and 28.3°C, and mean total precipitation of 27, 97 and 210 mm month-1.
In 2002 ambient temperature, relative humidity and global radiation were measured with the AIT weather station, whereas in 2003 temperature, relative humidity and global radiation were recorded at 1-min intervals outside and inside the greenhouse with a purpose-built computer system. Data for weekly means of temperature, relative humidity and radiation during both experiments are presented in figure 1.
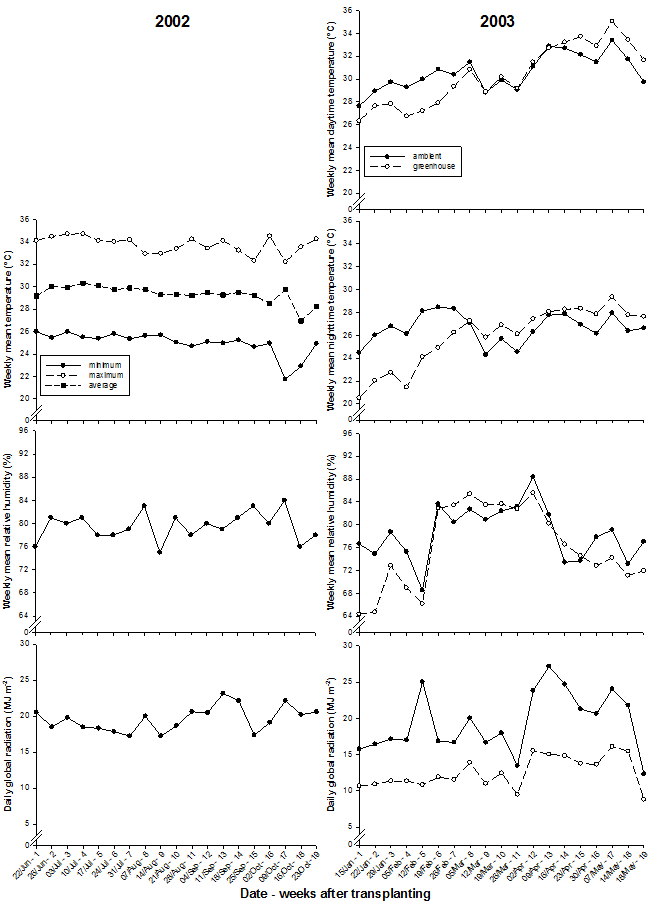
Fig. 1 Weekly means of ambient minimum, maximum and average temperature, relative humidity, and daily global radiation in 2002; ambient and greenhouse daytime and nighttime temperature, relative humidity, and daily global radiation in 2003.
Cultural practices
In 2002 and 2003, experiments were conducted in a non-cooled but ventilated polyethylene (PE) greenhouse (20 m long, 10 m wide, 7 m high). Materials for the roof were PE film and for the passively ventilated side walls, gables and 0.8-m-high openings at the roof ridge 42-mesh PE nets. Additional active ventilation was provided by two exhaust fans installed in one gable. These were turned on by the computer system when greenhouse temperature exceeded 25°C day and night and lowered the latter by ca. 1°C.
Seeds of indeterminate tomato (Lycopersicon esculentum Mill.) cultivar “King Kong 2” (Known-You Seed Co. Ltd, Kaohsiung, Taiwan) were sown on 22 May (experiment 1) and 20 December (experiment 2) 2002 in a nursery, and transplanted into 10–liter PE containers (30-cm in diameter) on 22 June 2002 and 15 January 2003, respectively. Substrate was a local commercial potting mix (31 % clay, 39 % silt, 30 % sand) with 0.40, 0.18 and 0.65 % total N, P and K (organic matter: 28 %) and a pH of 5.3. In the greenhouse, plants were distributed in either single rows or double rows with 30-cm distance within rows. Distances between single rows and centers of double rows were 160 cm and those between individual rows in double rows 40 cm. This resulted in plant densities of 2.1 and 4.2 plants m-2. Plants were grown at both densities either with single stems or double stems. The latter technique is recommended for field production during the hot-wet season in the sub-/tropics (Chen and Lal 1999). For these plants, one side shoot, which emerged from the first node below the first truss of the stem, was not pruned but allowed to develop into a second stem. Therefore, double-stem plants were composed of three stem parts, i.e., the (1) “base” stem up to the intersection between the (2) “primary” stem and the (3) “secondary” stem. Plants were trained according to the high-wire system (Van de Vooren et al. 1986) using “Bato” hangers (Bato Trading B.V., Zevenbergen, The Netherlands) attached to metal wires 4-m above ground with 160-cm distance between plants with a single stem and 40-cm distance between individual stems for plants with double stems. Cultivation practices followed those of intensive greenhouse production common in Northern and Central Europe including removing auxiliary shoots at weekly and “layering” at biweekly intervals (Van de Vooren et al. 1986). The plants were layered by releasing 30-60 cm string from the hangers and moving them ca. 30 cm along the high wires. Subsequently all plant parts, i.e., leaves and trusses, up to the bottommost truss with fruits were clipped. Tomatoes were fertilized and irrigated with a computerized fertigation system. Nutrients were injecting into the irrigation water at a rate of 0.1 % from two concentrated stock solutions. The first solution contained calcium nitrate (19 % Ca, 15.5 % N) and the second solution “Kristalon Orange” (6:12:36 % N:P:K) in 2002 and “Hakaphos basis” (3:15:36 % N:P:K) in 2003. Fertigation was scheduled based on a radiation sum of 0.4 kWh which was received up to 15 times per day. According to growth stage of plants, a volume of up to 0.4 l nutrient solution plant-1 was supplied during each irrigation cycle, resulting in a maximum of ca. 4.5 l plant-1 day-1 for mature plants. Other cultural practices were summarized by Katroschan (2003). Although fungal diseases could be successfully controlled under prevailing conditions of high air humidity and plant density, experiments were finalized at 19 WAT (weeks after transplanting) when plants were seriously damaged by tospovirus (e.g. capsicum chlorosis virus) vectored by thrips (e.g. Ceratothripoides claratris).
Experimental layout, sampling and analysis
Experiments in both 2002 and 2003 were laid out as factorial split-plot designs with three replications. The main-plot factor was “plant density” with two levels: “single rows” vs. “double rows” and the sub-plot factor “stem density” with two levels: “single stem” vs. “double stem”. There were six planting strips arranged lengthwise in the greenhouse of which the four treatment combinations were randomly assigned to the four central strips. These strips were subdivided into plots of three plants (plus one border plant) each when plant density was “single rows” and six plants (plus two border plants) each when plant density was “double rows”. Individual plants within plots represented replications. For non-destructive measurements, there were 18 replications (6 plots with 3 plants each) at the beginning and 3 replications (1 plot with 3 plants) at the end (19 WAT) of each experiment.
Plants were destructively measured (three replications) at approximately monthly intervals after transplanting by successively removing plots from one end of the greenhouse to the other. Biomass that was pruned during layering and harvest was measured when required. Fresh and dry weights (ventilated oven; 105°C for 48 h) from stem, individual leaves (including petioles), trusses (without fruits), and fruits were determined. Numbers of leaves (>3 cm), numbers of trusses (>3 cm) and numbers of fruits were recorded. Leaf area was measured with an area meter (LI-3100 from LI-COR Inc., Lincoln, USA). Yield was recorded as marketable yield after harvest in the greenhouse and as non-marketable yield after destructive sampling and layering. In 2003, additional non-destructive measurements in the greenhouse included number, position and spread of individual leaves and trusses within the canopy. This was done with an electromagnetic 3D digitizer (“FASTRAK”) from Polhemus (Polhemus Co., Colchester, USA). Digitized index points within the tomato canopy were (1) bases of vertical, non-layered stem parts, (2) internodes and (3) leftmost, rightmost and distal ends of leaves. From those data, length of internodes, and length and width of leaves were directly calculated whereas leaf area was extrapolated using a regression of destructively measured area on non-destructively measured leaf width:
LA = 0.8201*** × LW2; r2 = 0.59***
where: LA is leaf area and LW leaf width (***: significant at P < 0.001, n = 437). This confirms the good relationship between leaf width and area described by Schwarz and Klaring (2001).
Effects of experimental treatments were analyzed with split-plot analysis of variance (ANOVA) and means separated with the least significant difference (LSD) test. Other comparisons such as those between individual stems within the “double-stem” treatment were done with standard errors (SE). ANOVA, LSD and SE were calculated with appropriate procedures using the SAS System Version 8.02 (SAS Institute Inc., Cary, USA). Linear and non-linear regressions were carried out with SigmaPlot Version 8 (SPSS Inc., Chicago, USA).
Results
Climatic conditions
Although ventilation temperature was 25°C, the actual average greenhouse temperature during daytime was usually higher (Fig. 1). This was particularly true when ambient weekly mean daytime temperatures exceeded 32°C during the hot-dry season after the beginning of April 2003. Both outside and inside temperatures were on average 3-4°C lower at night than during day. Before 9 WAT, average ambient temperatures (daytime: 29.8°C, nighttime: 26.9°C) exceeded greenhouse temperatures (daytime: 28.0°C, nighttime: 23.7°C) whereas they averaged lower thereafter (ambient daytime: 31.2°C, nighttime: 26.4°C; greenhouse daytime: 32.0°C, nighttime: 27.6°C). Ambient relative humidity averaged 79 % in 2002 and 76 % in 2003, and average relative humidity inside the greenhouse was 78 % in 2003. Global radiation outside the greenhouse averaged 35.9 MJ m-2 and inside 23.7 MJ m-2, which is equivalent to a greenhouse transmission of 0.66.
Biomass accumulation and partitioning
In both 2002 and 2003, there was no significant effect of plant-density treatments on tomato biomass development on a per-plant basis. Therefore, productivity of double-rows was about twice that of single rows on a per-unit-area basis. Interactions between plant and stem density were not meaningful.
In 2002, double-stem plants produced 47 %, 30 %, 19 % and 35 % more stem, leaf, fruit and total biomass than plants with a single stem (Fig. 2). These differences were significant from 5 WAT. Accumulation of biomass during the 19-weeks cultivation period followed a sigmoid increase. Regressions of biomass parameters on time were highly determined and regression coefficients usually significant (Table 1). Biomass partitioning into generative organs was only a fraction of that into vegetative organs: without clearly changing with crop development, fruit dry mass accounted for less than 20 % of total dry mass whereas vegetative dry mass was greater than 80%. Leaf dry mass averaged at 42 % of total plant dry mass and stem biomass slightly less (39 %). Throughout the experiment, harvest produce was non-marketable with fruit fresh weight averaging 32 g fruit-1.
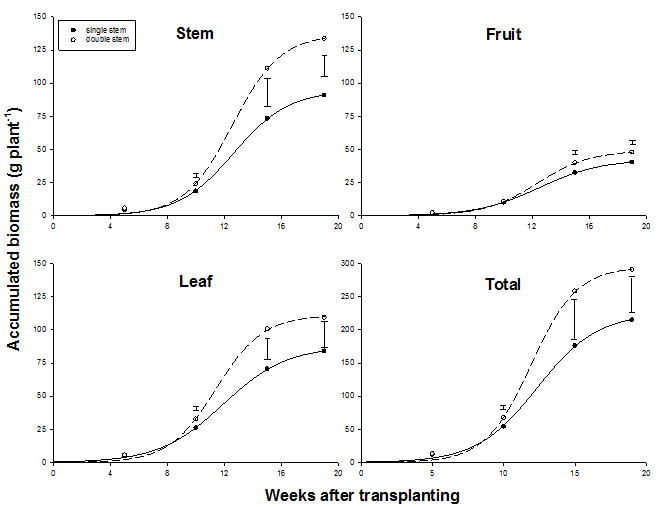
Fig. 2 Accumulated biomass of individual plant parts and accumulated total biomass (g dry mass plant-1) as affected by stem density in 2002. Error bars indicate LSD.
|
Table 1. Coefficients, r2 and levels of significance of regressions of accumulated biomass (g plant-1) of different tomato plant organs on cultivation period (WAT) as affected by stem treatments in 2002 a. |
|||||||||
|
Plant organ |
Function type |
Single-stem plants |
Double-stem plants |
||||||
|
|
|
a |
b |
x0 |
r2 |
a |
b |
x0 |
r2 |
|
Stem |
sigmoid b |
94.38* c |
1.928* |
12.63* |
>0.99* |
136.7* |
1.688* |
12.55* |
>0.99* |
|
Leaf |
sigmoid |
87.71* |
2.248* |
11.89* |
>0.99* |
111.1* |
1.652 n.s. |
11.41* |
>0.99* |
|
Fruits |
sigmoid |
42.43* |
2.172* |
12.49* |
>0.99* |
49.26* |
1.859 n.s. |
12.37* |
>0.99* |
|
|
|
|
|
|
|
|
|
|
|
|
Total |
sigmoid |
225.0* |
2.116* |
12.34* |
>0.99* |
295.3* |
1.612 n.s. |
11.93* |
>0.99* |
|
a See figure 2; b |
|||||||||
In 2003, biomass of trusses (without fruits) was only a small fraction of leaf biomass present on plants during the cultivation period (Fig. 3). As indicated by regression equations, leaf and truss biomass on the plant as well as biomass of leaf, truss, non-marketable and marketable fruits removed following layering and harvest, increased during early crop development, peaked at 11-14 WAT and decreased thereafter (Table 2). Differences between stem treatments were more pronounced during early crop development than later. An average of 36 % of leaf and truss biomass present on plants for single-stem plants and 26 % for double-stem plants was removed during each layering. These differences were due to greater leaf weight in plants with a single stem (see below). Non-marketable yield was more evenly distributed during the cultivation period than marketable yield since non-marketable fruits were harvested every 2-3 weeks during layering. The ratio between non-marketable and marketable fruit dry mass was 51:49 for single-stem plants and 45:55 for double-stem plants. Average fresh weight of non-marketable and marketable fruits was 18 g fruit-1 and 138 g fruit-1. Total fresh marketable yield on a per-unit-area basis averaged at 1.8 and 2.5 kg m-2 for single-stem plants and double-stem plants in single rows and 3.6 and 5.9 kg m-2 in double rows.
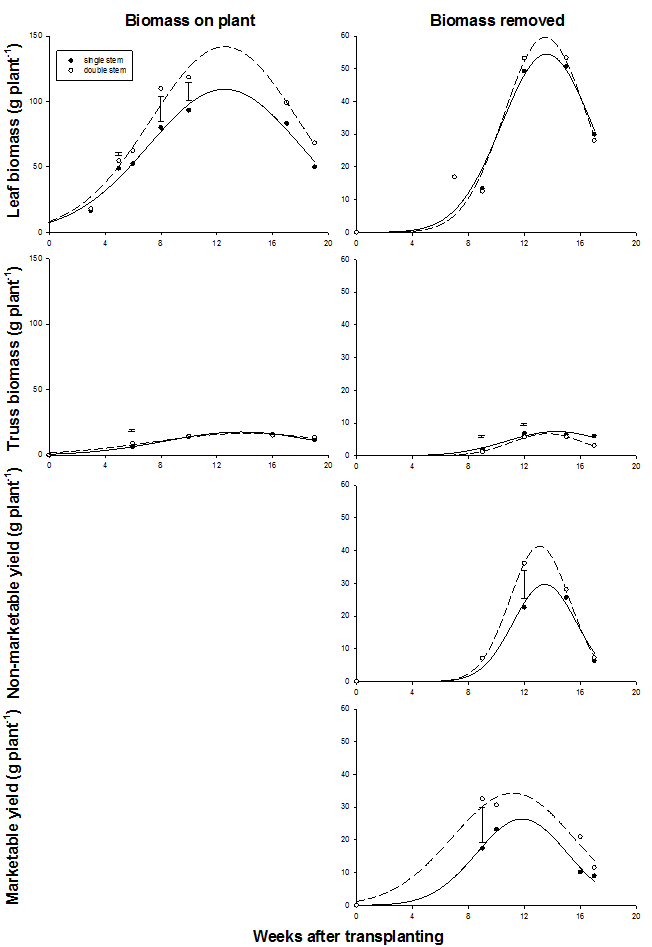
Fig. 3 Biomass on plants and removed biomass (g dry mass plant-1) as affected by stem density in 2003. Error bars indicate LSD.
|
Table 2. Coefficients, r2 and levels of significance of regressions of biomass on plants, biomass removed and accumulated biomass of tomato plant organs (g plant-1) on cultivation period (WAT) as affected by stem treatments in 2003 a. |
|||||||||
|
Plant organ |
Function type |
Single-stem plants |
Double-stem plants |
||||||
|
|
|
a |
b |
x0 |
r2 |
a |
b |
x0 |
r2 |
|
Biomass on plants |
|
|
|
|
|
|
|
|
|
|
Leaf |
peak c |
109.4*** d |
5.435*** |
12.55*** |
0.96** |
141.8*** |
5.265*** |
12.55*** |
0.96** |
|
Truss b |
peak |
17.75** |
5.473*** |
13.75*** |
>0.99** |
16.65** |
6.728* |
14.11** |
0.96* |
|
|
|
|
|
|
|
|
|
|
|
|
Biomass removed |
|
|
|
|
|
|
|
|
|
|
Leaf |
peak |
54.47** |
3.232** |
13.60*** |
0.93* |
59.61** |
2.946** |
13.49*** |
0.93* |
|
Truss |
peak |
7.428 n.s. |
3.557 n.s. |
14.28* |
0.89 n.s. |
6.747* |
2.609* |
13.634** |
0.99 n.s. |
|
Non-marketable yield |
peak |
26.69* |
2.272* |
13.45*** |
0.96* |
41.36*** |
2.138*** |
13.09*** |
>0.99*** |
|
Marketable yield |
peak |
26.38* |
3.235* |
11.82** |
0.98* |
34.27* |
4.330* |
11.17** |
0.97* |
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated biomass |
|
|
|
|
|
|
|
|
|
|
Stem |
sigmoid |
147.2*** |
4.002*** |
11.71*** |
>0.99*** |
275.1* |
5.592* |
14.31* |
0.98*** |
|
Leaf |
sigmoid |
195.1*** |
2.816*** |
8.522*** |
>0.99*** |
217.8*** |
2.251*** |
7.730*** |
>0.99*** |
|
Truss |
sigmoid |
34.32*** |
2.750** |
10.29*** |
>0.99*** |
31.66** |
3.266* |
9.820* |
0.99** |
|
Non-marketable yield |
sigmoid |
64.46*** |
1.544*** |
12.24*** |
>0.99*** |
78.51*** |
1.268** |
11.79*** |
>0.99*** |
|
Marketable yield |
sigmoid |
54.40** |
0.5570 n.s. |
9.420*** |
0.98* |
88.74** |
0.8565*** |
9.468*** |
0.98* |
|
|
|
|
|
|
|
|
|
|
|
|
Total |
linear |
|
17.00*** |
|
0.98*** |
|
25.72*** |
|
0.95*** |
|
a See figures 3 and 4;b
without fruits; c peak: d n.s.: not significant, *: significant at P< 0.05, **: significant at P<0.01, ***: significant at P<0.001 |
|||||||||
Cumulative biomass of tomato stem, leaf, truss, non-marketable yield and marketable yield could be modeled with sigmoid functions (Fig. 4, Table 2). Regression equations were mostly highly determined with highly significant coefficients. Except leaf biomass at 19 WAT and truss biomass after 6 WAT, biomass in plants with double stems accumulated to significantly greater amounts than in plants with single stems. Final total stem length in double-stem plants was 820 cm and in single-stem plants 432 cm. At the same time, total stem biomass of double-stem plants was 200 g and that of single-stem plants 128 g. Internode length averaged 6.1 cm with no differences between stem treatments. Single-stem plants produced a total of 70 internodes and double-stem plants 130 internodes. Differences in stem length between treatments were consequently due to differences in internode numbers rather than internode length. From these internodes developed a total of 54 leaves and 16 trusses in single-stem plants and 95 leaves and 35 trusses in double-stem plants. The surplus of trusses in plants with double stems was alleviated by lower numbers of fruits per truss (2.3) as compared to plants with single stems (3.7). Over all, individual stems extended ca. 26 cm with three new leaves and one inflorescence per week. Leaf weight averaged 3.7 g dry mass leaf-1 in single-stem plants and 2.2 g dry mass leaf-1 in double-stem plants whereas mean leaf area was 417 and 289 cm2 leaf-1, respectively. Although the average SLA tended to be slightly greater in plants with double stems (131 cm2 g-1) compared with single-stem plants (113 cm2 g-1), these differences were statistically not significant.
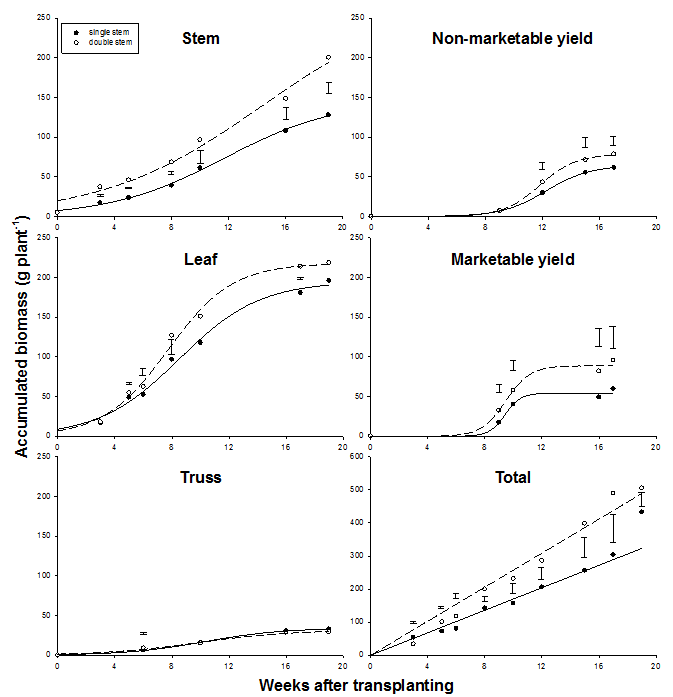
Fig. 4 Accumulated biomass of individual plant parts and accumulated total biomass (g dry mass plant-1) as affected by stem density in 2003. Error bars indicate LSD.
In contrast to the accumulation of biomass of individual plant parts, cumulative total biomass could be modeled with linear regression (Fig. 4, Table 2). The total of 433 g dry mass plant-1 in single-stem plants accumulated at a rate of 17.0 g week-1 whereas the total of 505 g plant-1 in double-stem plants accumulated at a rate of 25.7 g week-1. Plants with double stems had 56 %, 12 %, 60 % and 30 % greater stem, leaf, fruit and total biomass than single-stem plants. On average, 30% of total biomass was partitioned into stems and 38 %, 6 %, 13 % and 14 % into leaves, trusses, non-marketable and marketable fruits. 68 % of total biomass was partitioned into vegetative and the 32 % into generative organs. A greater portion of total biomass in single-stem plants was partitioned into leaves (41 %) than into the stem (27 %) whereas more biomass was partitioned into stems (35 %) than in leaves (32 %) in plants with double stems.
Figure 5 presents biomass accumulation and partitioning for individual stems of double-stem plants. Biomass accumulation of different plant parts as well as total biomass accumulation could be modeled with sigmoid regression (Table 3). The base stem contributed only little stem and leaf biomass (ca. 8 %) to total plant biomass. There were no significant differences between stem, leaf and truss biomass partitioned in either primary stem or secondary stem as indicated by SE. Compared to single-stem plants, growth rates of primary and secondary stems of double-stem plants were, therefore, equally reduced for these fractions. In contrast, differences between individual stems were significant for non-marketable and particularly marketable yield with much less fruit dry mass partitioned into the secondary than the primary stem. Total dry mass of non-marketable yield was only 64 % and that of marketable yield only 37 % of dry mass produced by the primary stem. The secondary stem produced only ca. 50 % of the total fruit biomass that was produced by single-stem plants. Although non-marketable yield produced by the primary stem was only 80 %, marketable yield was 120 % that of single-stem plants. Due to the differences in fruit biomass production, total biomass of primary stems was significantly greater than secondary stems after onset of removal of fruits by layering and harvest (9 WAT) except 16 and 19 WAT (Fig. 5).
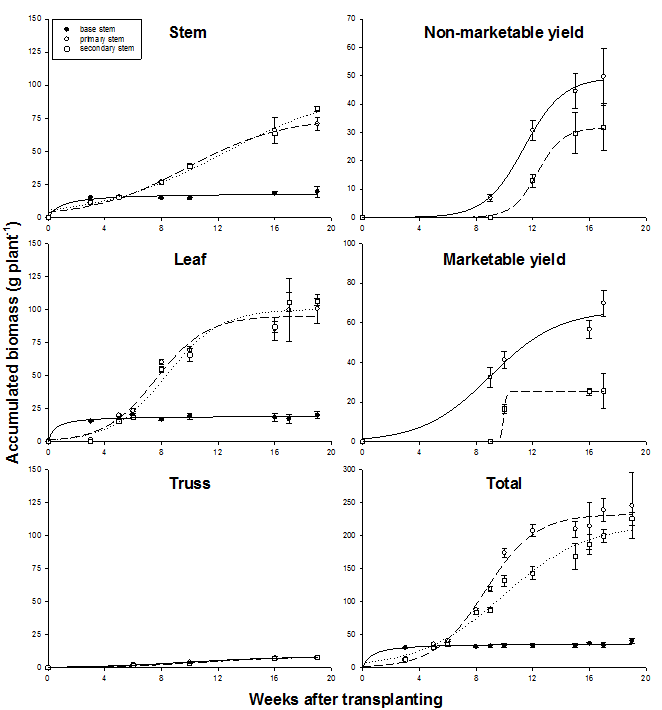
Fig. 5 Accumulated biomass of individual plant parts and accumulated total biomass (g dry mass plant-1) as affected by stem type in double-stem plants in 2003. Error bars indicate SE.
|
Table 3. Coefficients, r2 and levels of significance of regressions of accumulated biomass of tomato plant organs (g plant-1) on cultivation period (WAT) as affected by stem types of double-stem plants in 2003 a. |
|||||||||||
|
Plant organ |
Base stem |
Primary stem |
Secondary stem |
||||||||
|
|
a |
b |
r2 |
a |
b |
x0 |
r2 |
a |
b |
x0 |
r2 |
|
|
hyperbolic c |
sigmoid |
sigmoid |
||||||||
|
Stem |
18.45*** |
1.001 n.s. |
0.94*** |
75.79*** |
3.398** |
9.854*** |
0.99*** |
97.32** |
12.36** |
4.400** |
0.99*** |
|
Leaf |
19.56*** |
0.4862 n.s. |
0.95*** |
95.20*** |
1.790** |
7.620*** |
0.98*** |
100.3*** |
1.911** |
8.337*** |
0.98*** |
|
Truss b |
|
|
|
8.530** |
3.409* |
9.829* |
0.99* |
8.370** |
3.021* |
10.82** |
>0.99** |
|
Non-marketable yield |
|
|
|
49.35*** |
1.372* |
11.38*** |
>0.99** |
31.63*** |
0.8918* |
12.34*** |
>0.99*** |
|
Marketable yield |
|
|
|
66.41* |
2.395 n.s. |
9.008** |
0.97* |
25.43*** |
0.0995 n.s. |
9.938* |
>0.99*** |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
36.95*** |
0.6299 n.s. |
0.33 n.s. |
217.9*** |
2.933*** |
9.892*** |
0.98*** |
232.2*** |
1.778*** |
8.636*** |
0.98*** |
|
a See figure 5;b without
fruits; c hyperbolic: d n.s.: not significant, *: significant at P< 0.05, **: significant at P<0.01, ***: significant at P<0.001 |
|||||||||||
Discussion
As expected, global radiation available for plant growth was much greater in the tropical environment of Central Thailand (ca. 20-36 MJ m-2) compared with Northern Europe (ca. 3-15 MJ m-2, Heuvelink 1995a). In contrast to regions at greater latitude, sun position is much less important on affecting radiation than cloudiness: global radiation was 50 % lower during the hot-wet but cloudy “summer” season in 2002 compared with the hot-dry but sunny “winter” season in 2003. Greenhouse transmission was 0.66 and comparable with common greenhouse designs at greater latitudes (e.g. Critten 1987). Under the conditions of high radiation, dry-mass production averaged 2.6 and 2.7 g MJ-1 in 2002 and 2003 which is similar to tomato at greater latitude with much lower global radiation (2.5 g MJ-1, Heuvelink 1995a). Plant density had no significant effect on biomass accumulation since plants in double rows produced the same biomass on a per-plant basis and double the biomass on a per-unit-area basis compared with plants in single rows. This indicates that available photosynthetic active radiation (PAR) was not a limiting growth factor. Although data for interception of PAR by plants and transmission of PAR through the canopy are not presented in this paper, it can be concluded that plant density resulted in better interception of PAR on a per-unit-area basis and, therefore, increased availability of total assimilates for biomass production (Papadopoulos and Ormrod 1988a, Papadopoulos and Pararajasingham 1997). Typical responses of tomato to reductions in available radiation are longer internodes and greater leaf area (Papadopoulos 1991), none of which were observed in the studies presented here: plants in stands with lower stem density had greater leaf area than leaves of plants in stands with greater stem density. Although the latter had comparably greater SLA, these differences were statistically not significant. In contrast to other authors (e.g. Stoffella 1988, Saglam et al. 1995) no increase in number of fruits and decrease in fruit weight at greater planting and/or stem density could be measured. Therefore, the conclusion of Papadopoulos and Ormrod (1988a) that net photosynthesis of the younger top leaves of the tomato canopy may compensate for lower photosynthesis of the older and shaded leaves at the bottom of the canopy may be applicable to tomato under high-radiation conditions in the tropics.
The average CGR varied between 3-14 g dry mass m-2 day-1 according to season and experimental treatments. This rate is in-between rates (0-20 g m-2 day-1) documented for tomato at greater latitude during low-radiation conditions in winter and conditions of higher radiation in summer (De Koning 1989, Heuvelink 1995a). The ratio between number of leaves and trusses was 3:1 and truss appearance rate ca. 1 truss week-1 which is both in agreement with studies of De Koning (1989), De Koning (1994) and Heuvelink (1995a). 39-46 % of total plant biomass was distributed to stems, 42-43 % to leaves and 16-19 % to fruits. These averages are in sharp contrast to biomass partitioning in tomato under greenhouse cultivation in Northern Europe. Under the latter conditions, yield accounted for 84% of total fresh growth (De Koning 1989) and 54-60 % of cumulative total biomass (Heuvelink 1995a), and comparably less biomass was partitioned into stems (12-13 %) and more into leaves (28-33 %). SLA in our studies averaged 113-131 cm2 g-1 whereas SLA in the studies of Heuvelink (1995a) varied between 175-250 cm g-1 during summer and 300-400 cm g-1 during winter. In both of our studies, we observed that particularly older leaves at the bottom of the canopy were strongly curled and pointed downwards thus reducing light interception. This as well as decreases in SLA have been explained as negative feedback control on photosynthesis by small sink demand if sink demand is associated with number of fruits (or trusses) per plant (Heuvelink and Buiskool 1995, Heuvelink and Marcelis 1996). A number of authors have described mechanisms including accumulation of assimilates (Guinn and Mauney 1980), hormonal mechanisms affecting e.g. stomatal resistance (Gifford and Evans 1981) as well as mechanisms at the molecular level (e.g. Sonnewald and Willmitzer 1992) responsible for this phenomenon. Therefore, dry-mass partitioning in our studies might have been strongly affected by low sink-source ratio resulting from limited numbers of fruits. We also observed a trend towards excessive growth of auxiliary shoots, which can be explained as a reaction to increase sink strength and/or distribute accumulated assimilates towards vegetative plant parts (Heuvelink and Buiskool 1995).
Although growth of individual stems within double-stem plants was less than the growth of single-stem plants, there was no difference in partitioning of vegetative biomass between primary and secondary stem (Fig. 5). However, ca. 100 % more biomass was partitioned into fruits (120 g) in primary stems than in secondary stems (57 g) and marketable yield from primary stems even exceeded that from single-stem plants (96 g) by 20 %. It appears that presence of secondary stems primarily acted as an extra source of assimilates to be translocated into and thereby promote fruit growth in primary stems. This is supported by the studies of Heuvelink (1995c) who concluded that there is no resistance in assimilate transport between multiple stems in tomato plants. Better interception of the high PAR by increasing plant and stem density and thereby maximizing assimilation was, therefore, the premium measure to improve tomato fruit biomass under hot tropical conditions.
The most striking difference between our studies and other reports was the overall low percentage of total biomass partitioned into generative organs. This was particularly true during 2002 when development of all trusses proceeded under high-temperature conditions. Only 20 % of total biomass was partitioned to fruits and all fruits were non-marketable due to their small size and weight, and angular shape. These symptoms are characteristic for parthenocarpy, i.e., fruit development without pollination, which is closely related to high temperatures particularly during night (Adams et al. 2001, Sato et al. 2001). In 2003, 32 % of total biomass was partitioned into fruits and two early harvests (9-10 WAT, Fig. 4) yielded mostly marketable fruits with fresh weights up to more than 200 g fruit-1. These fruits were set during the cooler dry season ca. 3-6 WAT. In contrast, only few marketable fruits were harvested at the end of the cultivation period (16-17 WAT) which were set during the hot-dry season ca. 10-13 WAT. Besides reducing viability and longevity of pollen and therewith inducing parthenocarpy in fruits, high night temperatures during the hot-dry and hot-wet seasons accelerated respiration which could explain the comparably low CGR of tomato given the high PAR under our conditions. High air temperatures could be reduced to some extent by dense crop stands, i.e. high plant and stem density. At maximum CGR, leaf biomass and consequently transpiration up to 10 WAT (Fig. 4) in 2003, weekly mean daytime and particularly weekly mean nighttime temperatures were on average 1.8°C and 3.0°C below those measured outside the greenhouse (Fig. 1). However, when nighttime greenhouse temperatures exceeded ca. 27°C after 8 WAT, lack of pollination resulted in parthenocarpy and consequently low marketable yield. A management practice to improve tomato yields under high temperature conditions is the application of artificial growth regulators (auxins) such as CPA (chlorophenoxy acetic acid) and NAA (naphthylacetic acid) to flowers (Chen and Hanson 2001). This practice does not prevent early flower drop and parthenocarpy but improves enlargement of parthenocarpic fruits and is common practice in some countries in the sub-/tropics such as Taiwan.
Acknowledgements
We want to thank the German Research Foundation for funding, the Asian Institute of Technology for providing facilities and particularly Prof. Vilas M. Salokhe for his continuous support and valuable suggestions.
References
Adams, S., K. Cockshull and C. Cave 2001: Effects of temperature on the growth and development of tomato fruits. Ann. Bot., 88, 869-877.
Chen, J.T. and G. Lal 1999: Pruning and Staking tomatoes. AVRDC International Cooperators’ Guide, AVRDC pub # 9-490, Shanhua, Tainan: Asian Vegetable Research and Development Center.
Chen, J.T. and P. Hanson 2001: Summer tomato production using fruit-setting hormones. AVRDC International Cooperators’ Guide, AVRDC pub # 01-511, Shanhua, Tainan: Asian Vegetable Research and Development Center.
Critten, D.L. 1987: Light transmission and enhancement in greenhouses. Thesis, London: University of London.
Dayan, E., H. Van Keulen, J.W. Jones, I. Zipori, D. Shmuel and H. Challa 1993: Development, calibration and validation of a greenhouse tomato growth model: II. Field calibration and validation. Agric. Syst., 43, 165-183.
De Koning, A.N.M. 1989: Development and growth of a commercially grown tomato crop. Acta Hort., 260, 267-273.
De Koning, A.N.M., 1994: Development and dry matter distribution in glasshouse tomato: a quantitative approach. Thesis, Wageningen: Wageningen Agricultural University.
Gifford, R. and L.T. Evans 1981: Photosynthesis, carbon partitioning, and yield. Ann. Rev. Plant Phys. 32: 485-509.
Guinn, G. and J.R. Mauney 1980: Analysis of CO2 exchange assumptions: feedback control. In: Hesketh, J.D. and J.W. Jones (eds.) 1980: Predicting photosynthesis for ecosystem models III, Boca Raton, Florida: CRC Press, 1-16.
Heuvelink, E. 1989: Influence of day and night temperature on the growth of young tomato plants. Scientia Hort., 38, 11-22.
Heuvelink, E. 1995a: Growth, development and yield of a tomato crop: periodic destructive measurements in a greenhouse. Scientia Hort., 61, 77-99.
Heuvelink, E. 1995b: Effect of temperature on biomass allocation in tomato (Lycopersicon esculentum). Physiologia Plantarum, 94, 447-452.
Heuvelink, E. 1995c: Dry matter partitioning in a tomato plant: one common assimilate pool? J. Exp. Bot., 46, 1025-1033.
Heuvelink, E. and R.P.M. Buiskool 1995: Influence of sink-source interaction on dry matter production in tomato. Ann. Bot., 75, 381-389.
Heuvelink, E. and L.F.M. Marcelis 1996: Influence of assimilate supply on leaf formation in sweet pepper and tomato. J. Hort. Sci, 71, 405-414.
Jones, J.W., E. Dayan, L.H. Allen, H. Van Keulen and H. Challa 1991: A dynamic tomato growth and yield model (TOMGRO). Trans. Am. Soc. Agric. Eng., 34, 663-672.
Katroschan, K. 2003: Einfluss der Bestandesarchitektur auf die Ertragsphysiologie von Tomaten (Lycopersicon esculentum Mill.). Diplomarbeit, Hannover: Institut für Gemüse- und Obstbau, Abteilung Gemüsebau, Universität Hannover.
Kleinhenz, V. 1997: Technologies for sustainable vegetable production in the tropical lowlands. Ph.D. thesis, Technische Universität München, Lehrstuhl für Gemüsebau, München: Herbert Utz Verlag Wissenschaft.
Opeña, R.T., J.T. Chen, C.G. Kuo and H.M. Chen 1992: Genetic and physiological aspects of tropical adaptation in tomato. Adaptation of food crops to temperature and water stress: proceedings of an international symposium, Taiwan, 13-18 August 1992, Shanhua, Tainan: Asian Vegetable Research and Development Center.
Papadopoulos, A.P. and D.P. Ormrod 1988a: Plant spacing effects on light interception by greenhouse tomatoes. Can. J. Plant Sci. 68, 1197-1208.
Papadopoulos, A.P. and D.P. Ormrod 1988b: Plant spacing effects on photosynthesis and transpiration of the greenhouse tomato. Can. J. Plant Sci. 68, 1209-1218.
Papadopoulos, A.P. and D.P. Ormrod 1991: Plant spacing effects on growth and development of the greenhouse tomato. Can. J. Plant Sci. 71, 297-304.
Papadopoulos, A.P. and S. pararajasingham 1997: The influence of plant spacing on light interception and use in greenhouse tomato (Lycopersicon esculentum Mill.): a review. Scientia Hort., 69, 1-29.
Saglam, N., A. Yazgan, R. Fernandez-Munoz, J. Cuartero and M.L. Gomez-Guillamon 1995: The effects of planting density and the number of trusses per plant on earliness, yield and quality of tomato grown under unheated high plastic tunnel. Acta Hort., 412, 258-267.
Sato, S., M.M. Peet and R.G. Gardner 2001: Formation of parthenocarpic fruit, undeveloped flowers and aborted flowers in tomato under moderately elevated temperatures. Scientia Hort., 90, 243-254.
Schwarz, D. and H.P. Kläring 2001: Allometry to estimate leaf area of tomato. J. Plant Nutr. 24, 1291-1309.
Sonnewald, U. and L. Willmitzer 1992: Molecular approaches to sink-source interactions. Plant Phys., 99, 1267-1270.
Stoffella, P.J., S.J. Locascio, P.H. Everett, T.K. Howe, J.W. Scott and S.M. Olson 1988: Yields of two tomato cultivars differing in shoot growth at several plant populations and locations. HortScience 23, 991-993.
Stützel, H. and W. Aufhammer 1991: Light interception and utilization in determinate and indeterminate cultivars of Vicia faba under contrasting plant distributions and population densities. J. Agric. Sci., 116, 395-407.
Van de Vooren, J., G.W.H. Welles and G. Hayman 1986: Glasshouse crop production. In: Atherton, J.G. and J. Rudich (eds.) 1986: The tomato crop. A scientific basis for improvement. London: Chapman and Hall, 581-623.
Received October 05, 2004 / Accepted October 27, 2005
Addresses of authors: Volker Kleinhenz (corresponding author – present address), FieldFresh Foods Pvt. Ltd., Golf View Corporate Towers, 6th Floor, Sector – 42, Gurgaon 122001, India, and K. Katroschan and F. Schütt, Asian Institute of Technology, ASE/SERD, Klong Luang, Thailand, and H. Stützel, Hanover University, Faculty of Horticulture, Institute of Vegetable Production, Hanover, Germany, e-mail: v.kleinhenz@gmail.com.
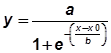 ;
c n.s.: not significant, *: significant at P< 0.05, **:
significant at P<0.01, ***: significant at P<0.001
;
c n.s.: not significant, *: significant at P< 0.05, **:
significant at P<0.01, ***: significant at P<0.001