Sulfur and Chloride in the Soil-Plant System
Kleinhenz, V., 1999
K + S Group, Kassel (International Potash Institute, Basel). pp. 99
Sulfur and Chloride in the Soil-Plant System
Dr. agr. Volker Kleinhenz
K+S
Aktiengesellschaft
Bertha-von-Suttner-Straße 7
D-34111 Kassel
GERMANY
Contents
|
1 Introduction |
|
|
2 Sulfur and Chloride in Soils |
|
|
2.1 General |
|
|
2.2 Soil sulfur |
|
|
2.2.1 Total soil sulfur |
|
|
2.2.2 Fractions of S in soil |
|
|
2.2.3 Transformations of S in soil |
|
|
2.2.4 Availability of S in soil |
|
|
2.3 Soil chloride |
|
|
2.4 The salt index of fertilizers |
|
|
3 Absorption of Sulfur and Chloride by Plants |
|
|
3.1 Soil conditions in the rhizosphere soil |
|
|
3.2 Root growth |
|
|
3.3 Ion uptake |
|
|
3.4 Interactions between nitrogen, sulfate and chloride |
|
|
3.5 Interactions between absorption of sulfur/chloride and other nutrients |
|
|
3.6 Salt tolerance of plants |
|
|
3.6.1 Salt tolerance at germination |
|
|
3.6.2 Salt tolerance after germination |
|
|
4 Sulfur and Chloride in Plants |
|
|
4.1 Translocation of sulfate and chloride |
|
|
4.2 Sulfur assimilation |
|
|
4.2.1 Sulfate activation and reduction |
|
|
4.2.2 Incorporation of S into organic forms |
|
|
4.3 S-containing organic compounds |
|
|
4.3.1 Sulfide bond |
|
|
4.3.2 Disulfide bond |
|
|
4.3.3 Rhodanide |
|
|
4.3.4 Heterocyclic S compounds |
|
|
4.3.5 Classification of S-containing organic compounds |
|
|
4.4 Effects of S supply on organic compounds and quality of crops |
|
|
4.4.1 Effects on photosynthesis |
|
|
4.4.2 Effects on amino acids and proteins |
|
|
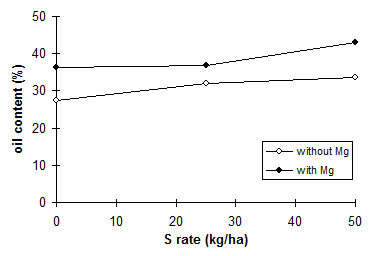
4.4.3 Effects on lipids (oils and fats) |
|
|
4.4.4 Effects on sugars |
|
|
4.4.5 Effects on starch |
|
|
4.4.6 Interactions between S and other nutrients |
|
|
4.5 Functions of chloride in plants |
|
|
4.6 Chloride tolerance |
|
5 Summary |
|
|
5.1 Sulfur and Chloride in Soils |
|
|
5.2 Absorption of Sulfur and Chloride by Plants |
|
|
5.3 Sulfur and Chloride in Plants |
|
References |
Secure food supply is a basis for economic, social and cultural development, and for political stability. To match future food demand, food production must be dramatically increased. It is projected that the world population will increase to 8.2 billion people by the year 2025, a 53-percent increase from 1990. A majority of the future world population will live in tropical regions such as sub-Saharan Africa and South Asia with more than 50 percent in Asian countries (von Uexküll, 1995).
Increased food production may only be achieved by intensification of production on existing agricultural land (Kleinhenz, 1997). This will widen the gap between removal of plant nutrients by crop uptake and nutrient release in soils by mineralization. To close the gap, nutrients must be applied in the form of fertilizers or manures. The more intensive the crop production, the more likely is the use of inorganic fertilizers.
Sulfur deficiency is becoming widespread and is emerging as an important constraint in crop production worldwide. S is now recognized as the fourth major plant nutrient and it can be expected that with increasing intensification of agriculture, demand for S will soon outstrip supply. This is particularly true for the Asian countries like India (Biswas & Tewatia, 1991), Bangladesh (Bhuiyan, 1991), Thailand (Chaiwanakupt et al. 1987), China (Liu et al. 1993), the Philippines (Mamaril et al. 1991), Indonesia (Ismunadji, 1991), and the South Pacific Islands (Morrison et al. 1987). There are multiple reasons for S deficiencies such as increased crop yield levels through breeding, better control of S emissions from industrial and domestic fuel burning, use of low-S high analysis fertilizers, and decreasing applications of organic manures and S-containing pesticides. Potassium sulfate (sulfate of potash, K2SO4) is not only a good source of immediately plant-available S for the numerous crops worldwide, but also has a number of advantages over the usual and more cheaply available K fertilizer, potassium chloride (muriate of potash, KCl): (1) it carries two major plant nutrients, (2) it has a lower salt index, (3) it is preferable for chloride-sensitive crops, and (4) it improves quality of many crops.
Chloride is considered a micronutrient since most plants require only trace amounts of Cl to meet their physiological requirements. However, for some species Cl is essential for maintaining certain processes. More commonly, chloride is associated with detrimental effects on soil salinity, salt intolerant crops, and crops which a vulnerable to Cl-toxicity.
This booklet is an update of information contained in: Zehler et al. (1981): Potassium Sulphate and Potassium Chloride but focuses on (1) the processes which sulfur and chloride are subject to in soils, (2) processes which determine availability of sulfur and chloride and their absorption by plants, and (3) physiological and metabolic functions of sulfur and chloride in plants. In these veins, it incorporates the latest available information on production of a large number of crops to provide examples for the processes which sulfur and chloride undergo in the soil-plant system, their functions in plants and how this affects productivity and quality of agricultural produce, and sustainability of agricultural land.
2 Sulfur and Chloride in Soils
The soil is a complex system of heterogeneous material in which nutrients are present in different fractions. These pools of nutrients depend on the chemical and mineralogical properties of soils and their parent materials but can be altered, e.g. through fertilizer application.
The total content of a nutrient in soil is usually only a poor indicator of availability of this nutrient since only a small percentage is directly accessible to plants. The nutrient pool that can be absorbed by plant roots, the available nutrient fraction, is usually analyzed as the concentration of exchangeable ions in the soil liquid phase, the soil solution. Although the soil solid phase is the main reservoir for nutrients, the liquid phase is responsible for nutrient transport to the absorbing plant root. Plants deplete the nutrient concentration from the adjacent soil and thereby create a sink towards nutrients, which diffuse along a negative gradient. Absorption of nutrients by plants is greater and therefore diffusion of nutrients towards their roots generally faster the higher the nutrient concentration in the soil solution (Mengel & Kirkby, 1987).
Several interactions exist between nutrient pools and they are usually described as nutrient transformations or nutrient cycles. The concentration of H+ ions in the soil solution is expressed in terms of pH as a measure of potential acidity and has a pronounced effect on transformations, and therefore pool size of soil nutrients. Environmental conditions (e.g. humid or arid climatic conditions) and cultivation techniques (e.g. paddy field cultivation) modify availability of plant nutrients directly and indirectly through altering soil pH conditions. Native chemical and mineralogical properties of soils, environmental conditions, and cultivation practices determine the pool size and extent of transformations of sulfur and chloride in soils and, therefore, their availability to plants. Greater or smaller availability of these nutrients can exert positive or detrimental effects on plant growth and crop performance.
Sulfur in soils principally originates from the pyrite (FeS2) of primary minerals. During weathering and soil formation, the S from pyrite is oxidized to several forms of different states, which are intimately connected with the soils reduction-oxidation potential. Oxidation and reduction reactions of S occur rather easily, accounting for the diversity of reactions that S undergoes in the soil.
The total S content of soils varies substantially: According to Tandon (1991), the total S concentration of soils in India ranges from 19 ppm to 9750 ppm. If 10 ppm S equals 20 kg S/ha (Biswas & Tewatia, 1991), the total S content in those soils ranges from 38 to 19,500 kg/h. A total-S content of 15-60 kg/ha may be common in soils. Total S is usually related to the following soil properties: (1) organic matter content, (2) clay percentage and type of clay minerals, (3) content of active iron and aluminum oxides, and (4) soil texture.
(1) Content of organic S in soil increases with its humus content. Particularly peat and marsh soils are rich in organic S. In the South Pacific total S tends to be less in soils with low organic matter content (Morrison et al.1987). The highly weathered and leached tropical soils are typically low in organic matter and S (Stevenson, 1986). (2) In soils of South China, total S is positively correlated with clay percentage (Liu, 1986). Total S is higher when kaolinite (1:1) type clay minerals are dominant relative to montmorillonite (2:1) type clays (Morrison et al. 1987). (3) S retention in soils is favored by the presence of iron and aluminum oxides (Shin, 1987). (4) In soils of India total S is higher in fine textured soils than in coarse textured soils, partly because of their greater organic matter content (Tandon, 1991).
The total content of S in soils is usually only a weak indicator of availability of S to plants. Plants mainly absorb S in the form of SO42-. However, soil S occurs in several forms and, therefore, the availability of S to plants depends on the transformations of S in the soil and the interactions between the different S fractions. Figure 1 provides a rough estimate of distribution of different fractions of S in soils.
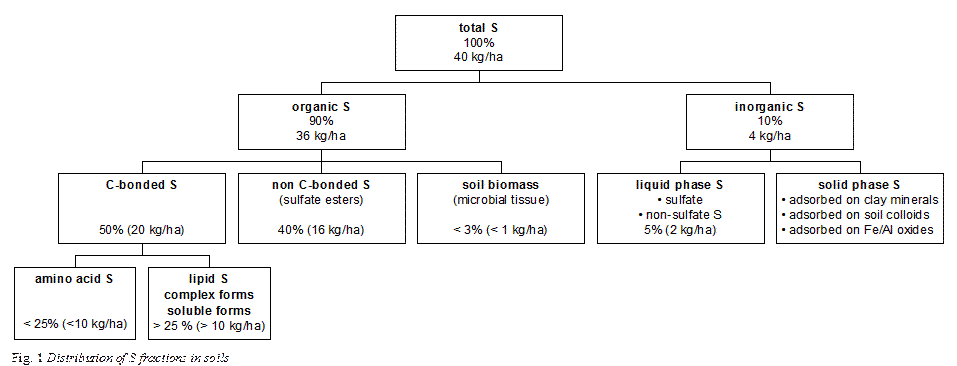
Organic S
Although the proportion of organic S can vary greatly (e.g. from 5% to 98% in Indian soils; Takkar, 1988) this fraction dictates the total S content of most soils (Blair et al.1991). The distribution of organic S within a soil profile follows the pattern of organic matter and decreases with depth. Soil organic S can be divided into three fractions: C-bonded S, non C-bonded S, and the soil biomass (Stevenson, 1986; Figure 1).
The greater part of total organic S in soils of humid and semiarid regions are present as C-bonded S. Separation of this fraction is difficult since these S-containing compounds undergo extensive transformations. Although it is anticipated that S present as amino acid S accounts for only a minor part of the carbon-bonded S, trace quantities of this fraction can be identified in soil whereas for the other forms (lipid S, complex forms, and soluble forms) it is usually possible only to demonstrate their occurrence. The S-containing amino acids include cysteine, cystine, and methionine. However, only a small fraction of the C-bonded S can be accounted for these known compounds. The greater part occurs as lipid S (sulfolipids), complex S-forms, and soluble S-forms. These forms occur as numerous products resulting from other complex compounds and do only exist in soil as intermediate products between synthesis and destruction by microorganisms.
Non C-bonded S is assumed to occur as unknown ester sulfates, such as sulfated polysaccharides. Only a small part of the organic S resides in soil biomass. Nevertheless, the fraction of S in microbial tissue is extremely labile and responsible for the turnover of S in soil with consequences for the availability to plants.
Inorganic S
Inorganic S occurs in soil largely as SO42-. S is available to plants as sulfate in the liquid phase of the soil. Under anaerobic conditions, S is present in reduced forms (Table 1). A major fraction of the S in calcareous and saline soils occurs as gypsum (CaSO4·2H2O). In arid regions, high amounts of salts such as CaSO4, MgSO4, and Na2SO4 can accumulate.
Solid phase S comprises SO42- retained in an adsorbed form. Sulfate can be adsorbed to clay minerals and active Fe and Al oxides. Sorption is due primarily to anion exchange by positive charges on clay minerals and oxides and increases with decreasing soil pH.
|
Table 1 Important forms of inorganic soil sulfur |
||
|
Oxidation state |
Sulfur form |
|
|
-2 |
Sulfide Sulfide Hydrogen sulfide |
S2- H2S |
|
-1 |
Polysulfide Pyrite |
FeS2 |
|
0 |
Elemental Sulfur |
S0 |
|
+2 |
Thiosulfate |
|
|
+4 |
Sulfite |
|
|
+6 |
Sulfate |
|
|
|
Sulfate |
|
|
|
Sulfuric acid |
H2 |
2.2.3 Transformations of S in soil
Since organic S provides the major S reservoir in most agricultural soils, organic S transformations are of great importance. For plant nutrition, the strictly microbiological process of conversion of organic S forms (including organic residues) to inorganic, plant available forms (mineralization) is particularly important. S from the different organic fractions is converted to inorganic S by a diversity of partly unidentified microorganisms. The type of transformation and its end product depend on (1) the organic S substrate to decompose and (2) the reduction-oxidation status (aeration) of the soil.
Inorganic S transformations are ultimately decisive for the availability of S to plants: the oxidized and plant-available SO4-form can be assimilated by microorganisms (immobilization) and incorporated into microbial tissues (assimilatory reduction). SO42- can be reduced to (e.g.) hydrogen sulfide by heterotrophic microorganisms (dissimilatory reduction). On the other hand, autotrophic microorganisms can oxidize reduced forms of S to SO42-.
The quantity of plant-available SO42- in the soil solution is the difference between the magnitude of the (organic and inorganic) transformation processes. There are three particular agricultural environments, which modify the relative importance of these transformations substantially: (1) acid, tropical soils; saline, alkaline soils; and (3) flooded soils. Fertilizers containing different forms of S alter availability of S and other plant nutrients in those environments.
Organic S transformations
Figure 2 presents general pathways of organic S transformations:
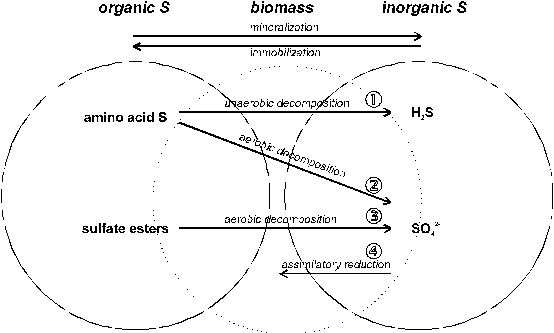
Fig. 2 Organic S transformations (see text)
Several valid pathways have been shown to exist for the anaerobic decomposition of S-containing amino acids in soil. One example is the formation of H2S from cysteine:
H2O
HSCH2CH(NH2)COOH à CH3COCOOH + NH3 + H2S
Cysteine Pyruvic acid
‚ Oxidation of amino acids to plant available SO42- proceeds in several ways since a diversity of microorganisms is involved. One example is the stepwise oxidation of cysteine to sulfate:
O2
HSCH2CH(NH2)COOH à HOOSCH2CH(NH2)COOH
Cysteine Cysteine sulfinic acid
O2
HOOSCH2CH(NH2)COOH à HOOOSCH2CH(NH2)COOH
Cysteine sulfinic acid Cysteic acid
O2
HOOOSCH2CH(NH2)COOH à SO42-
Cysteic acid Sulfate
ƒ For decomposition of sulfate esters as a major source of organic S in soils, the enzyme arylsulfatase has been found responsible. The enzyme has been detected in soils from several geographical regions.
„ Assimilatory SO42- reduction describes the process of incorporation of S into cellular organic constituents (immobilization).
Inorganic S transformations
Figure 3 presents transformation processes of inorganic forms of S:
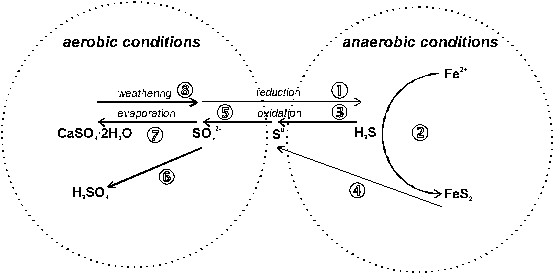
Fig. 3 Inorganic S transformations (see text)
Under anaerobic soil conditions, SO42- is reduced to sulfide. This dissimilatory SO42- reduction is carried out by at least two strict anaerobe genera of bacteria, Desulfovibrio and Desulfotomaculum. In waterlogged soils H2S may accumulate to toxic levels and impair plant growth.
‚ H2S is a strong reducing agent in soil. The S from sulfide reacts with reduced ferrous Fe2+ to form insoluble metal sulfides such as pyrite (FeS2). Pyrite can get lost through volatilization. Precipitation of these sulfides can cause pollution of soil and natural water. In flooded soils, this process is accelerated and can limit the availability of both S and Fe.
ƒ Oxidation of reduced forms of S is carried out by chemotrophic and photosynthetic bacteria. Chemotrophic colorless sulfur bacteria (Beggiatoa, Thiothrix, Thiobacillus) oxidize H2S to elemental S. They retrieve energy from this oxidation process to reduce carbon dioxide for carbohydrate production. Photosynthetic colored bacteria exist under anaerobic soil conditions (e.g. rice fields). For these bacteria, H2S is electron donator for reduction of CO2 in photosynthesis. Oxidation of reduced-S fertilizers is generally slow and does not satisfy crop demand (Germida, 1990)
„ FeS and FeS2 can be oxidized in a partly biological (Thiobacillus thiooxidans) and partly chemical process to form elemental S. These reactions are closely connected with formation of H2SO4 (see †).
… Chemotrophic (Thiobacillus) and heterotrophic (Actinomycetes) bacteria oxidize elemental S0 to SO42- (Germida, 1990). The effect of S oxidation is to lower the soil pH and may have beneficial effects under conditions of calcareous and saline soils. However for neutral or acid soils, the acidifying effect is undesirable. Repeated applications of S0 to some Canadian soils decreased activity of microbial biomass and soil enzymes (arylsulfatase) liberating S from the organic matter (Gupta et al. 1988). The long-term implication had been a reduced turnover of organic matter and other nutrients. By contrast, populations of sulfur oxidizers were stimulated. With the exception of strongly acid soils elemental sulfur fertilizers should only by applied to correct substantial soil S deficiency.
† Both oxidation of H2S and FeS2 results in formation of H2SO4 which can result in extremely acid soils:
H2O + O2
FeS2 à FeSO4 + S
H2O + O2
S à H2SO4
Drainage of wetland areas and use of elemental S can create serious problems with soil acidity when agriculturally used. Acid sulfate soils are widespread in low-lying areas, particularly in the tropics.
‡ Due to excessive evapotranspiration in arid and semi-arid regions inorganic salts accumulate in the upper soil layer. Such halomorphic soils contain high amounts of S and can be divided into saline soils and alkaline soils. Sulfur accumulates in saline soils as sulfates of Ca, Na and Mg. At a later stage, alkaline soils develop as hydrolysis of Na2CO3 and NaHCO3 releases OH- ions thus resulting in high pH conditions.
ˆ Application of gypsum is a common practice for improving saline and alkaline soils. Besides its positive effects on soil structure, the neutral Ca salt replaces Na+ on the sorption sites of soil colloids.
Adsorption of S
Inorganic S can be present in soil solution (liquid phase S) or adsorbed on soil colloids (solid phase S). SO42- in soil solution is in equilibrium with the S in solid phase forms. Sorption of SO42- is due primarily to anion exchange by positive charges on Fe and Al oxides (Figure 4) and clay minerals. SO42- is probably adsorbed specifically (adsorption by ligand exchange; ionic exchange of SO42- for surface OH- ions) and unspecifically (adsorption to protonated groups). Therefore, sulfate is not as strongly retained in soil as phosphate, which is largely adsorbed by ligand exchange.
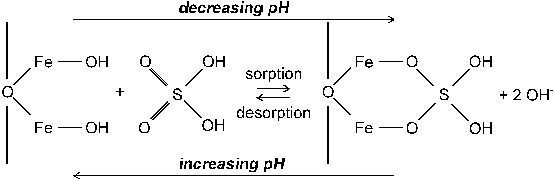
Fig. 4 Sulfate adsorption by ligand exchange. With decreasing pH sulfate
is adsorbed at the surface of a Fe oxide
Anion adsorption decreases in an order: phosphate >> sulfate > chloride = nitrate. Sulfate adsorption capacity of clay minerals follows the sequence: kaolinite > illite > betonite. This adsorption is greater in the subsoil than in the topsoil: Hähndel & Isermann (1993) measured large amounts (600 kg/ha for sandy soils to 7,000 kg/ha for loamy sands) of SO42- in the subsoil of intensive vegetable fields.
Adsorption of sulfate is pH dependent, being favored under low pH conditions. When a soil is limed, the H+ concentration in the soil liquid phase is lowered. Since soil liquid phase and soil solid phase are in equilibrium, H+ ions are removed from the colloidal surface, thus creating surface negative charges. Because of the lower amount of net positive charge at higher pH, SO42- retention will diminish (Figure 5).
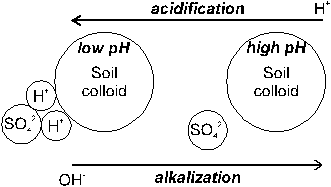
Fig. 5 Adsorption and desorption of sulfate on soil colloids
2.2.4 Availability of S in soil
S is absorbed by plants in the form of SO42-. Therefore, availability of S in soil depends on the concentration of SO42- in the soil liquid phase. Environmental factors and agricultural practices together with soil-related factors determine the availability of S for plants.
Mineralization of organic S
Mineralization of S from organic matter is not directly related to soil type, contents of C and N, or soil pH. On peat soil, most plant available S will be supplied from organic matter (Nurzynski et al. 1980). However, Stevenson (1986) concluded that the S content of recently added plant residues regulates the amount of SO42- mineralized and not the total content of organic S in soil. Organic S may be more resistant to mineralization than organic C and N (Freney, 1986). Fox & Hue (1986) stated that S mineralized from organic matter might be only a low percentage of that of N mineralization. Long-term application of potassium sulfate may have a positive effect on the soil organic matter content: (Yanishevskii et al. 1990) stated that moderate application rates of potassium sulfate increased humus and total N after 30 years in Russia. However, large amounts of K2SO4 had the opposite effect. Soil conditions like high soil temperatures and sufficiently high soil moisture which stimulate growth and activity of decomposing microorganisms will affect the mineralization of organic S. Turnover rates of sulfur are, therefore, particularly high in the humid tropics. Similar to organic nitrogen, mineralization of organic sulfur is accelerated by alternate drying and re-wetting of the soil (e.g. during irrigation cycles), but decomposition proceeds slower under saturated conditions of flooded soils (Zhu et al. 1984). There is no equilibrium between S in organic matter and in the soil solution. In tropical climates with distinct dry and rainy seasons, Reynolds-Vargas et al. (1994) and Kleinhenz et al. (1997) observed accumulation of nitrate-nitrogen in the course of the dry season. This may also be true for sulfate-sulfur. In general, there is a positive correlation between organic matter content and available S in soils. However, this trend is more pronounced in humid regions where plant-available SO42- leaches easily and does usually not accumulate in soil.
S in tropical soils
Among others, three environmental factors are reason for the oftentimes low availability of S in tropical soils: (1) High temperature conditions in the humid tropics are conductive to a rapid mineralization of organic matter. Organic matter and organic S in agricultural soils are therefore usually low. (2) High rainfall conditions accelerate leaching of plant available SO42-. (3) Acid tropical soils may have some capacity to retain sulfate.
Although its content in soils of humid, tropical regions is low, availability of S in these areas depends substantially on soil organic matter: Liu (1986) found a highly significant positive correlation between soil organic matter and soil S in southern China. Compared with a soil in northern China, a soil in southern China was much lower in total S but only a small percentage of this S derived from the inorganic pool (Table 2).
|
Table 2 Concentration and approximate content of sulfur in two soils of China |
|||||||||
|
Soil |
Total S |
|
Inorganic S |
|
Organic S |
|
|||
|
|
ppm |
kg/haa |
|
% of total S |
kg/ha |
|
% of total S |
kg/ha |
|
|
Loess soil in northern China |
226 |
454 |
|
39.4 |
179 |
|
60.6 |
275 |
|
|
Red soil in southern China |
146 |
292 |
|
7.3 |
21 |
|
92.7 |
271 |
|
|
a 1 ppm » 2 kg/ha, Source: Liu (1986) |
|||||||||
In Thailand (Parkpian et al. 1986), no significant relationship was found between sulfate S and organic matter in lowland soils where leaching is restricted due to high water tables. In contrast, good correlation was obtained for well-drained upland soils. These soils are particularly low in S and usually respond favorable to sulfur applications (Chaiwanakupt et al. 1987).
Highly weathered tropical soils low in organic and inorganic soil colloids have a low buffer capacity for H+ ions. Therefore, these soils readily develop acidity which is the H+ concentration of the soil solution and the concentration of H+ ions adsorbed to soil colloids. High H+ concentrations favor the exchange of H+ in the soil solution for cations adsorbed to soil colloids. Toxicity to plants is not the pH per se but high concentrations of aluminium ions displaced from clay minerals, which are toxic to plant roots of many species. Due to a greater number of H+ ions on the colloids, their surface positive charge is increased and SO42- retention favored (Blair & Lefroy, 1987). Great quantities of sorbed sulfate can accumulate in acid soils. As much as 16 tons SO4-S/ha have been reported in some soil profiles of the tropics (Fox & Hue, 1986). However, this adsorbed sulfate is only sparingly soluble and available to plants. In Hawaii, a soil low in adsorbed sulfate was in equilibrium with a greater concentration of SO42- in soil solution compared with a soil high in adsorbed sulfate (Table 3).
|
Table 3 Relation of adsorbed sulfate to sulfate in the soil solution in two Hawaiian soils |
||
|
Soil property (0-100 cm) |
Soil type |
|
|
|
Mollic Vitrandept |
Hydric Dystrandept |
|
adsorbed SO42- (mg/kg) |
118 |
627 |
|
soluble SO42- (mg/l) |
5.0 |
1.8 |
|
Source: Fox & Hue (1986) |
||
In acid soils, when H+ replaces other cations (Na+, K+, Mg2+, Ca2+) from colloids, sulfate can be leached as the accompanying counter-ion, adding acidity to the surface soil (Kennedy, 1986). This may explain why S can be high in the solid phase but at the same time low in the liquid phase. Adsorbed sulfate usually accumulates only in the subsoil where pH is lower and content of clay and Fe and Al oxides usually higher. This S may be taken up by deep-rooted plants (Bohn et al. 1986) but plants intolerant to Al toxicity develop only shallow root systems in the oxidized surface soil.
S in saline, alkaline soils
In arid and semi-arid regions, elemental sulfur is readily oxidized. By end of the oxidation process, sulfuric acid is formed which reacts with CaCO3 and calcium phosphates to form CaSO4 or gypsum. Since concentration of H+ ions in the soil solution is increased upon oxidation of S, these hydrogen ions exchange for other cations adsorbed to soil colloids. Therefore, the availability of many plant nutrients like phosphorus, iron, manganese, and zinc is increased upon the reduction in pH of alkaline soils (Mostafa et al. 1990). Abd-Elfattah et al. (1990) measured the amount of total soluble salts after application of elemental S to an alkaline clay soil: (1) in an initial phase (two months) the EC of the soil quickly increased after application and declined with time. (2) Until four months from S-application, the EC decreased to the level recorded before application. (3) After four months the salt content declined below that level indicating the beneficial effect of sulfur application on the salinity of soils (Hilal, 1990). This decline was attributed to enhanced leaching of salts.
S in flooded soils
One of the most important agricultural activities is the production of rice on wetland soils. Within a flooded soil, there are aerobic and anaerobic zones. The aerobic zones are restricted to a thin oxidized surface soil layer and an oxidized layer surrounding the plant root. Both oxidation and reduction reactions can occur simultaneously in those different parts of the flooded soil (Figure 6). Similar to the transformations of N, the mineralization rate of organic S is low in flooded soils (Blair & Lefroy, 1987). By contrast, immobilization of SO42- into organic S is high: more than one third of added inorganic sulfate may be immobilized, 10 percent more than under aerobic conditions (Sachdev & Chhabra, 1974).
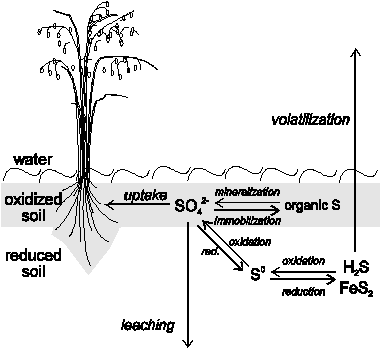
Fig. 6 S transformations in flooded rice soils
Elemental S from fertilizers is only slowly oxidized and frequently not recovered by an initial crop. However, such fertilizers usually exert long residual effects on subsequent crops (Mamaril et al. 1991). Availability of S in flooded soils is further restricted by the reduction of SO42- to sulfide in reduced soil layers, which can be precipitated by iron, manganese, and other heavy metals or volatilized. The existence of active metal ions in flooded soils may reduce the negative impact of H2S on rice roots (e.g. in Korea; Shin, 1987). Similarly, oxidation of elemental S to sulfate may be negatively affected in reduced soil layers. Reduced S may act as a reservoir of S for aquatic crops because it may re-oxidize in the rhizosphere of crops (Blair, 1986).
Interactions between S and other nutrients in soil
Availability of anions such as SO42- in soil solution depends on the existence of equivalent amounts of counter-cations such as Ca2+, Mg2+, Na+, and K+. Therefore, availability of sulfate does also depend on the concentration of cations in the soil solution. In the oxidation of reduced S species to SO42-, hydrogen ions are produced which can release other cations by exchange from soil colloids. These cations may play a role in balancing SO42- in soil solution. However, cations like Ca2+ may obscure availability of SO42- by formation of insoluble species such as CaSO42-. The addition of CaCO3 can lead to an increase in soluble SO42-. This may be due to release of adsorbed SO42- because of the increase in soil pH (Stevenson, 1986).
Interactions between sulfate and phosphorus have been studied by several authors. This interrelationship may be due to competition for anion adsorption sites in soil. The adsorption strength of PO43- is expected to be higher than for SO42-. Dressings of fertilizer P may therefore result in desorption of SO42- since PO43- substitutes sulfate on the adsorption sites (Pasricha & Aulakh, 1990; Abd-Elfattah et al. 1990). This may increase availability of S to plants but makes S at the same time more vulnerable to leaching. Santoso (1989) found higher recovery rates of S when the S fertilizer was applied in combination with P fertilizer (Table 4).
|
Table 4 Effect of P fertilizer on recovery of fertilizer S in corn |
|
|
Fertilizer application |
Recovery of S in fertilizer (% of applied S) |
|
S separated from P |
2.7 |
|
S mixed with P |
12.4 |
|
Source: Santoso (1989) |
|
However, heavy application rates of S-free P fertilizer can result in heavy leaching of S into the subsoil (von Uexküll, 1986). Adetuni (1992) studied some Nigerian soils and warned of S deficiencies following application of phosphate.
Some authors have mentioned the effect of lime (CaO) on availability of SO42-. The principal effect of lime is to react with H2O to form calcium hydroxide which neutralizes free H+ in the soil solution. Adsorbed SO42- is released to the soil liquid phase and therefore made available. This may be desired to neutralize acid soils (Marscher et al. 1992). However, in high rainfall tropical climates released sulfate will be subject to rapid loss through leaching (Adetuni, 1992).
Application of elemental sulfur to alkaline soils usually increases the availability of other nutrients. This is due to the acidifying effect of oxidation of S to sulfuric acid. By lowering the pH, S can increase availability of P on high pH, calcareous soils (von Uexküll, 1986). Availability of micronutrients such as Fe, Zn, and Mn is augmented upon the acidifying effect of oxidation of elemental S in high-pH soils (Abdel-Samad et al. 1990; Zhu & Alva, 1993). The effect of S oxidation on the availability of these nutrients is to lower the oxidation-reduction potential of the soil and increase their solubility by reducing them (e.g. insoluble Fe3+ is reduced to soluble Fe2+).
Effects of sulfur on physical and biological properties of soils
Particularly in soils of arid regions, application of sulfur can significantly improve their physical and biological properties. Hilal (1990) and Reddy et al. (1978) showed that the fine particles of S increase the water holding capacity of desert soils (Table 5). Hilal attributed this to the electrical neutrality of the S0 particles, which cut the capillary rise of soil moisture and thereby act as an evaporation barrier. Sulfur may decrease soil hardness by weakening the bondings of soil colloids and breaking down CaCO3 (reacts with sulfuric acid to form CaSO4 or gypsum) concentrations in soil (Table 5). Crust strength of clays and surface sealing of soils can be reduced with S (So et al. 1978).
|
Table 5 Effect of sulfur application on moisture and hardness (0 to 30-cm depth) after three years in an Egyptian soil |
||
|
S rate |
soil moisture (%) |
soil hardness (penetrometer reading) |
|
S0 |
1.59 |
266 |
|
S1 |
2.60 |
217 |
|
S2 |
2.74 |
161 |
|
Source: Hilal (1990) |
||
Chloride is quickly removed in the process of weathering of soils. Therefore its content in soils is usually low (von Uexküll & Sanders, 1986). Chloride may accumulate in coastal regions, in arid and semi-arid zones, and when high doses of Cl-containing fertilizers are applied. Most Cl- occurs as salts such as NaCl, MgCl2, and CaCl2. Cl- concentration in soils is usually low (0.5 to less than 35 ppm) but can accumulate in saline soils to substantial levels (possibly more than 6000 ppm; Tisdale et al. 1985).
Chloride can inhibit nitrification of NH4-N in moderately acid soils (Christensen et al. 1986). By stabilizing the NH4 concentration in soil, the population of Mn oxidizers is reduced (Beaton et al. 1988). The availability of soil Mn is further improved by triggering Mn release from soil colloids due to complexing of Mn2+ to MnCl (Krishnamurti & Huang, 1987). Cl was found to mobilize cadmium in soil (Salardini et al. 1993): in the absence of leaching availability of Cd to opium poppy increased. When evaporation exceeds rainfall/irrigation in arid and semiarid regions chloride can be transported towards the surface through capillary rise and can accumulate in soil. KCl is faster soluble in water compared with K2SO42-. This aspect seems to makes potassium chloride more suitable for fertigation systems (e.g. Elam et al. 1995). However, such installations are usually used only under arid conditions and for high-value crops (e.g. vegetables). High soil Cl concentrations and the susceptibility of many such crops to Cl make K2SO4 the preferred potassium source for fertigation systems.
Leaching of sulfate and chloride
When added to the soil, the different ions from fertilizers dissolve in soil solution. Understandably, their concentration in the soil liquid phase increases. Soil liquid phase and soil solid phases are in equilibrium. There is some potential for sulfate to be adsorbed in soils (see Chapter 2.2.3). At a soil low in solid-phase S, a greater portion of externally added SO42- will be adsorbed. However when solid-phase S is high, a smaller portion of added SO42- will be adsorbed and is therefore available to plants but, at the same time, vulnerable to leaching loss. This is particularly pronounced when added SO42- exceeds the soils capacity to adsorb S: all sulfate will remain in the soil liquid phase. There are two reasons why the Cl- anion is more rapidly leached than the SO42- anion: (1) since the Cl- anion has a great ion size it is only weakly (unspecifically) adsorbed, (2) the monovalent Cl- ion is more hydrated in solution than the SO42- ion (Saurat & Boulay, 1985). Therefore, chloride is much more readily leached than sulfate. At the same time, the potassium in KCl-fertilizer is more readily leached than in K2SO4-fertilizer (Figure 7). Application of sulfur to chloride-affected soils may improve leaching of Cl: 50 percent less water was required to leach Cl salts to an appreciable level in a desert zone (Hilal, 1990). Only in very acid (< pH 5) soils (e.g. in the tropics) Cl- can be adsorbed to soil colloids (e.g. iron oxide), particularly in the subsoil (Halstead et al. 1991).
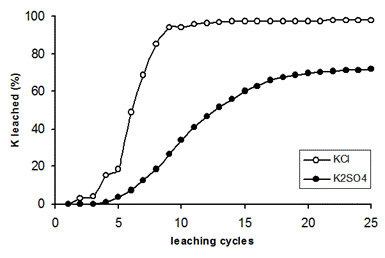
Fig. 7 Leaching of potassium from KCl and K2SO4 fertilizer (Source: Suarez-Vasques & Carillo-Pachon, 1984)
2.4 The salt index of fertilizers
Fertilizers increase the ion concentration in the soil solution and can harm plant root cells. This is particularly pronounced in saline and alkaline soils which already contain high amounts of soluble salts. Another effect of the high ion concentration of the soil solution on plants is the elevated osmotic pressure that binds soil water through the hygroscopic effect of the ions. This renders soil water less available to plant roots.
White & Ross (1939) recognized that a given rate of different fertilizers has a varying effect on the concentration of the soil solution. Rader et al. (1943) defined the salt index as a measure of the effect of different fertilizers on the concentration of the soil solution. This index is defined as the ratio of the increase in osmotic pressure produced by the material to that produced by the same weight of sodium nitrate (Table 6).
|
Table 6 The salt index of some potash fertilizers compared with sodium nitrate a |
||
|
Potash fertilizer |
Percentage of K2O in material |
Salt index |
|
Potassium chloride |
60.0 |
116.3 |
|
Potassium nitrate |
46.6 |
73.6 |
|
Potassium sulfate |
54.0 |
46.1 |
|
Sulfate of potash-magnesia |
21.9 |
43.2 |
|
a Percentage of N in material: 16.5; salt index = 100 Source: Rader et al. (1942) |
||
With regard to the effect on the salinity of soils, sulfate fertilizers are clearly superior to the potash fertilizers containing chloride as the counter-ion. Soil moisture exhibits a large effect on the salt concentration of soils. Therefore, potassium sulfate is usually the preferred fertilizer in arid or semi-arid regions, and under dry conditions such as in greenhouses (von Peter et al. 1995; Rasool et al. 1987; Davide et al. 1986). This is also true for soils with a low moisture-holding capacity (e.g. sandy soils; Nabi et al. 1990).
3 Absorption of Sulfur and Chloride by Plants
Chapter 2 refers to processes in the bulk soil. However, conditions at the interface of absorbing plant root and soil (rhizosphere) are sometimes considerably more different from those distant from the roots. At least three factors determine the nutrient availability to plants: (1) soil conditions in the bulk soil, (2) soil conditions in rhizosphere soil, and (3) root growth. Point (1) has been outlined in Chapter 2.
3.1 Soil conditions in the rhizosphere soil
For some time, it has been argued that plant roots absorb soil nutrients by an exchange process as they elongate in soil and come in contact with ions (interception theory). The amount of ions which directly contact plant roots is, however, small and two other processes, (1) mass-flow and (2) diffusion from bulk soil to rhizosphere soil were found the most important processes governing nutrient absorption by plants (Mengel & Kirkby, 1987).
(1) Mass-flow is the process when solutes are passively transported with soil water from soil to plant roots. Absorption thus depends on the rate of water flow and the nutrient concentration of the soil water. (2) Diffusion is the transport of ions from a higher to a lower concentration in soil solution. Roots deplete the nutrient concentration in their near vicinity and nutrients diffuse towards the low concentration (sink) surrounding the root surface. Both processes are responsible for the availability of nutrients to plants but it is recognized that diffusion plays a major role in absorption.
Nutrients present at a high concentration in the soil solution are mainly transported by mass-flow whereas nutrients in low concentrations are moved by diffusion. The rate of mass-flow depends on the solution concentration and the transpiration rate of the plant. Diffusion is generally greater in soils with higher nutrient levels and therefore steeper concentration gradients. Diffusion is greater for mobile ions than for those which can be adsorbed by soil colloids. Availability of such ions to plants embraces a quantity component and an intensity factor of how strongly the ion is retained in soil. In this respect the rate of diffusion of the Cl--ion is greater than the diffusion rate of SO42-. Sufficient soil moisture is a prerequisite for diffusion. As soils dry out diffusion is drastically reduced and poor nutrient mobility may become a more significant growth-limiting factor than the direct effect of deficient soil water. With decreasing soil moisture, the solution becomes more concentrated. However, concentrations of individual ions in the soil solution vary widely (Table 7). Particularly Cl-, Na+, SO42- and Mg2+ accumulate to high concentrations in salt affected soils.
|
Table 7 Ion concentrations in water saturated soil |
|
|
Element |
Range (mM) |
|
Cl |
0.2-230 |
|
Na |
0.4-150 |
|
S |
< 0.1-150 |
|
Mg |
0.7-100 |
|
N |
0.16-55 |
|
Ca |
0.5-38 |
|
K |
0.2-10 |
|
P |
< 0.001-1 |
|
Source: Fried & Shapiro (1961) |
|
Root exudation
Besides excretion of polysaccharides, roots exude organic compounds such as amino and organic acids. These compounds considerably increase populations of decomposing microorganisms in the soil rhizosphere. The N2 fixing bacteria Nitrobacter and Nitrosomas are two prominent examples.
The plant rhizosphere contains larger and more diverse populations of heterotrophic microorganisms and may be a potentially important site for oxidation of S (Germida, 1990). Refat et al. (1990) showed that the solubility of SO42- was dramatically higher in the rhizosphere of barley than in the bulk soil. This was more significant for a sandy soil compared with a clay loam soil. They attributed this to an accumulation of sulfur oxidizing bacteria, which actively produce SO42- in the rhizosphere of plants. Different crops seem to stimulate different populations of S oxidizers. Therefore, the efficiency of S fertilizers may be affected by the type of crop grown (Germida, 1990).
Root morphology comprises root depth, root branching, number of root hairs and tips, etc. Despite influences of plant genetics and other environmental factors (e.g. soil aeration, soil hardness), the nutrient distribution in soil determines root elongation in soil. Roots of plants often accumulate in zones with optimal concentration of nutrients. Too low or too high nutrient concentrations may restrict root growth.
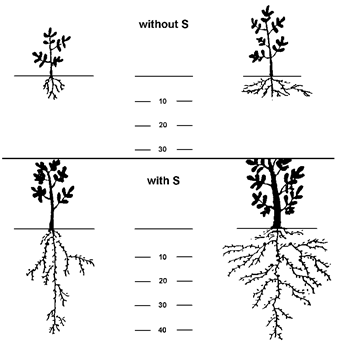
Fig. 8 Effect of sulfur application on root distribution of lupines
(Source: Hilal, 1990a)
Several studies show the effect of elemental sulfur on root growth of plants in arid zones and alkaline soils. Due to the acidifying effect of S oxidation, availability of other nutrients (e.g. P) was increased (Hilal et al. 1990b) and root penetration significantly increased (Figure 8; Hilal et al. 1990a). A similar trend was found for maize supplied with both calcium nitrate and K2SO4 (Luisi et al. 1983).
It is obvious that such deeper root systems have favorable effects on water uptake of crops under limited water supply. Growth and yield of numerous crops was significantly improved by sulfur application to alkaline soils in arid agroecosystems.
Absorption of different ions by plants can be described by a hyperbolic relationship between the content of nutrients in the soil medium and in the plant (Figure 9).
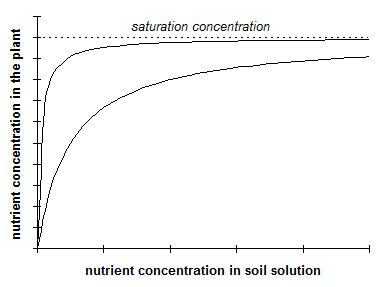
Fig. 9 Hyperbolic relationships between nutrient supply and absorption by
plants
This model assumes that at a low concentration of the nutrient in the soil medium this nutrient is effectively absorbed. With increasing concentration in the soil, the efficiency of the plant to absorb the nutrient decreases. The concentration of the nutrient in the plant cannot exceed a specific saturation concentration. For example, Kleinhenz (1997) estimated the saturation concentration of nitrate in plant sap of leafy vegetables at 10,000 ppm. The appearance of the Michaelis-Menten curve may differ widely with nutrient species, crop species, and environment. Unfortunately, data for regression of concentrations of plant SO4 (Cl) on soil SO4 (Cl) are lacking. Halstead et al. (1991) mentioned the potential of soil and plant tests for predicting crop response to chloride application in semi-arid and sub-humid areas of North America. However, the model may not be valid for absorption and assimilation of sulfate. For N, it is assumed that the rate of uptake of nitrate coincides with the rate of assimilation of N into organic compounds. This may not be true for S: the maximum rate of assimilation of inorganic S is attained earlier than the maximum of absorption of sulfate (see Chapter 4.1). Therefore, the presence of inorganic SO42- in plants may already indicate sufficient supply of S.
According to the principle of charge neutrality, the total charge concentration in the soil solution and in biological cell compartments is zero. Therefore, when plant root cells assimilate ions, the external and internal charge must be balanced. Soil cations like K+ are mainly absorbed passively. The membrane-bound enzyme ATPase splits ATP into ADP + Pi to provide energy for the uptake process. H+ ions are released from the cytoplasm (ion pumps) into the rhizosphere soil to build up a negative charge at the inner side of the cell membrane. Thus, cations at the outer side of the membrane are attracted into the root cell. Excreted H+ protons balance the resulting negative charge in the rhizosphere soil. Besides this electrical component, absorption of cations depends also on concentration gradients. With passive absorption, the concentration of cations in the root cell cannot exceed the soil solution concentration. Cation uptake is ion-specific since membrane activity differs widely for the various cation species.
For anion uptake, a negative charge must be transported into the negatively charged root cell. For this reason, it has been supposed that anions such as SO42- and Cl- are mainly absorbed actively by root cells in exchange for OH- or HCO3- (Mengel & Kirkby, 1987). Excretion of cytoplasmatic hydroxyl ions and absorption of anions outside the root cell is mediated by an anion carrier (Figure 10).
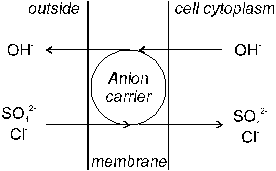
Fig. 10 Active absorption of SO42- and Cl-
anions by an anion carrier (antiport mechanism; Source: Mengel & Kirkby, 1987)
Such carrier systems obviously possess specific binding sites for anions and are, thus, anion-specific (Kennedy, 1986). Whether ATPases are involved in this process is unclear. Plant root cells are always negatively charged and therefore anions are more subject to active transport than cations. According to this antiport mechanism of uptake, two hydroxyls may be excreted for one sulfate anion assimilated, and for the chloride anion one.
Another uptake mechanism has been proposed for the absorption of Cl- (and possibly SO42-; Mengel & Kirkby, 1987). According to this symport theory (Sanders, 1984) an ATPase proton pump transports H+ ions to the outside of the root cell and a symporter transports two H+ for each Cl- into the cell cytoplasm (Figure 11). Energy for these processes derives from the ATP ADP + Pi reaction. Therefore, Cl- uptake is enhanced by illumination.
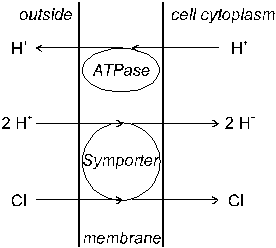
Fig. 11 Active absorption of Cl- anions by a symporter (symport
mechanism; Source: Sanders, 1984)
One important effect of active absorption is that anions can be absorbed against concentration gradients and cell anion concentrations can exceed concentrations in soil many times. This is particularly true for chloride (Figure 12).
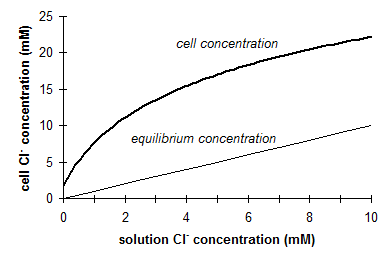
Fig. 12 Chloride concentration in mungbean root tips compared with
solution concentration (Source: Mengel
& Kirkby, 1987)
Sulfur is mainly absorbed in the highest oxidation form as SO42-. Although it may be possible for plant roots to absorb S-containing amino acids, this may have no practical implications for plant nutrition. It has been proven that amino acids can be transported into cells by specific ATPase-driven carriers. Sulfate uptake may not be very pH sensitive, nor is it significantly influenced by other elements (von Uexküll, 1986). However, selenate, which is chemically related to SO42-, may depress sulfate uptake substantially. Both ions probably compete for the same carrier sites in the process of active absorption. Application of sulfate may offset toxic effects of Se in alkaline soils in which it can accumulate.
Cations and anions are absorbed by plants at different rates. Cl- is much faster absorbed than SO42- and the speed at which the cation K+ in potassium fertilizers is absorbed depends on the accompanying anion (Table 8).
|
Table 8 Cation and anion uptake of young barley roots as influenced by potassium source |
||||
|
Potassium source |
Cation uptake |
|
Anion uptake |
|
|
(1 me/l) |
(m e/g) |
|
||
|
K2SO4 |
17 |
|
< 1 |
|
|
KCl |
28 |
|
29 |
|
|
Source: Hiatt (1967) |
||||
K+ from potassium chloride is obviously faster assimilated than K+ from potassium sulfate. This is probably not due to different effects on the availability in soil: Brunet & Treto (1988) mentioned that application of KCl and K2SO4 had similar effects on the K fractions in soil. Marsh et al. (1992) showed that leaf K concentrations in kiwi were higher with KCl than with K2SO4. Jackson & McBride (1986) measured the concentration of potassium in petioles of potatoes after application of potassium chloride and potassium sulfate. Results indicate that the K from potassium chloride is faster assimilated than from potassium sulfate (Table 9). Plants grown in single nutrient solutions containing equivalent concentrations of K as KCl or K2SO4 generally take up more K from the KCl solution. One reason is that the monovalent Cl- is absorbed more rapidly than the bivalent SO42- (Beringer & Mutert, 1991). El-Leboudi et al. (1993) attributed this to a different number of K-uptake mechanisms: K uptake from KCl by potatoes used three uptake mechanisms whereas K uptake from K2SO4 used only two. However, this is only a weak argument to promote KCl as a potassium source in potato production since the Cl anion has a detrimental impact on the quality of tubers (Chapter 4).
|
Table 9 Effect of potassium fertilizer on petiole K concentrations in potato |
|
|
Potassium fertilizer |
Petiole K concentration (%) |
|
None |
6.5 |
|
K2SO4 |
8.4 |
|
KCl |
9.6 |
|
Source: Jackson & McBride (1986) |
|
Ionic imbalances and effect on soil pH by absorption of KCl and K2SO4
Application of KCl has no effect on the pH of the soil. However, it is known that application of potassium sulfate lowers the pH in the rhizosphere of crops (Römheld, 1983).
For absorbing one cation K+, plants must excrete one H+ ion to neutralize the negative charge of the soil solution. According to the antiport theory of absorbing anions, OH- ions must be excreted to neutralize a positive charge in the soil solution.
Absorption of K+ and Cl- from KCl occurs at similar rates. Therefore, plant roots do not need to excrete H+ ions to balance anions in the soil solution and produce organic acids to balance anions in plant cells (Hiatt, 1967). Therefore, absorption of KCl has no effect on soil pH.
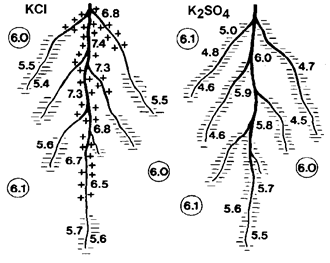
Fig. 13 Change in rhizosphere pH of maize after application of KCl and K2SO4
(Source: Römheld, 1983)
Several factors such as root respiration (release of CO2), release of protons or organic acids by roots, and differences in uptake of cations and anions can modify the pH of the soil rhizosphere (Römheld, 1983). Kennedy (1986) argued that absorption of sulfate may induce a temporary alkalization of the soil rhizosphere. Lin (1981) showed that decreasing the pH of the rhizosphere soil solution may therefore have a stimulating effect on the uptake of sulfate. However, the overall acidifying effect of K2SO4 application may be primarily due to the imbalances in absorption of cations and anions (Table 9): K+ from potassium sulfate is absorbed at a much faster rate than SO42- so that cation uptake greatly exceeds anion uptake. If uptake of cations exceeds uptake of anions, more anions remain in the soil solution and the pH temporarily increases. The cation-anion imbalance in the soil solution is compensated by excretion of H+ ions from plant roots into the soil solution. The efflux of protons is responsible for the frequently observed drop in pH after application of potassium sulfate to cropped soil: H+ protons substitute K+ cations in the rhizosphere soil and produce acidity. It follows that the excess in cation uptake results in acidification of the root environment (Figure 13). Drastic changes in pH can be observed in the rhizosphere zone of plant roots, particularly near growing points (Kennedy, 1986). Some acidification of the rhizosphere has been regarded beneficial for solubilizing essential nutrients such as phosphates. Fortunately, K2SO4 has only a slight and timely limited effect on decreasing the soil pH (Horsnell, 1985). The efflux of H+ protons from roots cells is compensated by production of organic acids at a rate equal to the rate of K+ uptake. The greater the deficit in anions relative to cations the greater the accumulation of organic acids (e.g. malate) in the roots. Thus, plants fed with potassium sulfate contain high amounts of organic acids. For the synthesis of organic acids, CO2 is needed. Therefore, absorption of K2SO4 triggers assimilation of CO2 much more than KCl. The overload of positive charge in root cells is compensated by negatively charged carboxylate (Figure 14). These compounds (e.g. calcium oxalate) are often stored in cell vacuoles.
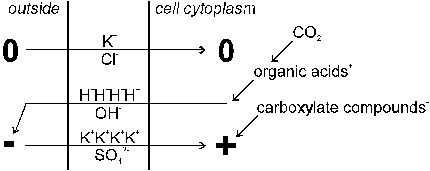
Fig. 14 Balancing charges in rhizosphere soil and root cells during
absorption of K+ cations, and Cl- and SO42-
anions from: (top) potassium chloride and (bottom) potassium sulfate
3.4 Interactions between nitrogen, sulfate and chloride
A very common fertilizer mix not only in tropical crop production is ammonium sulfate ((NH4)2SO4) together with KCl. For non-wetland crop production there may be a need to substitute ammonium for nitrate-nitrogen and substitute KCl for K2SO4.
Most non-wetland crops (e.g. vegetables) absorb nitrate-nitrogen considerably more rapidly than ammonium nitrogen (Scarsbrook, 1965). Ammonium nutrition can be harmful for susceptible crops (e.g. Chinese cabbage Ikeda, 1991). Moreover, oxidation of ammonium to nitrate invariably contributes significant acidity to soil (Kennedy, 1986). In intensive vegetable production in tropical regions, the overuse of ammonium sulfate can have dramatic consequences over the long term. In Taiwan, Huang et al. (1989) concluded that the overuse of fertilizers is the main reason for acidification of soils in one major vegetable production zone. The originally neutral soils (pH 6-7) in this region have changed to slightly to strongly acid (pH below 5.5). Similar observations have been made in Sri Lanka (Table 10).
|
Table 10 Effect of ammonium sulfate on soil pH |
|||
|
Rate |
Soil pH (0-15 cm) |
||
|
(kg/ha×year) |
1955 |
1966 |
1970 |
|
168 |
4.80 |
4.31 |
4.04 |
|
336 |
4.80 |
3.36 |
3.79 |
|
Source: Amarasiri (1987) |
|||
By mistake, farmers in Korea frequently associate the acidifying effect of ammonium sulfate to the sulfur component (Shin, 1987). It follows that particularly for intensive crop production (e.g. vegetable production) in which commonly great amounts of fertilizers are used, ammonium sulfate should be avoided. As a source for S, Salim & Rahmatullah (1986) pointed out ammonium sulfate and potassium sulfate was equally effective in mustard.
Therefore, when leaching is negligible or can be controlled, nitrate fertilizers may be the better option for N-supply, and K2SO4 the better choice to supply crops with potassium and sulfur: (1) the Cl in potassium chloride can exert detrimental effects on soils (inhibition of nitrification see below, salinity Chapter 2.4) and susceptible crops (Chapter 4). (2) Possible toxicity of ammonium (Barker & Mills, 1980) can be avoided. (3) Plants fed with nitrate contain higher levels of cations and organic anions but less chloride. For example, Miley & Oosterhuis (1993) found higher yields with sulfate and nitrate in cotton. Table 11 shows the favorable effects of nitrate nutrition on K uptake (van Beusichem & Neeteson, 1982; von Braunschweig, 1988), production of organic acids, and reduction in Cl assimilation. Apparently, ammonium represses the uptake of other cations. This can have significant consequences for the quality of crops both positive and negative (Chapter 4).
NO3
81
221
25
25
162
239
NH4
40
141
25
31
54
136
Source: Kirkby (1968)
The competitive effect in uptake between Cl- and NO3- as well as Cl- and SO42- is well known (e.g. in potatoes Muraka et al. 1973). Chloride can reduce uptake of nitrate. Goos et al. (1987a) and Goos et al. (1987b) reported a reduction in nitrate assimilation of barley by application of KCl (Table 12). Wehrmann & Hähndel (1984) found that chloride application decreased the nitrate concentration in spinach tops.
|
Table 12 Effect of KCl fertilization on the nitrate-nitrogen concentration in barley |
|
|
KCl rate (kg/ha) |
NO3-concentration in barley (ppm NO3-N) |
|
0 |
3934 |
|
50 |
2413 |
|
200 |
1804 |
|
Source: Goos et al. (1987a) |
|
Chloride can have an inhibiting effect on nitrification in moderately to strongly acid soils (Christensen et al. 1986). Reductions in nitrate uptake by chloride may also be due to competitive inhibition of the nitrate enzyme carrier system at the root surface. Those effects may be valuable for decreasing excessive nitrate levels in food crops but may interfere with optimal crop nutrition. K2SO4 may improve both, nitrate assimilation and reduction in plants (Chapter 4) thereby increasing biomass production and decreasing nitrate levels at the same time. Therefore, particularly in intensive vegetable production in oftentimes acid tropical soils, potassium sulfate should be preferred to potassium chloride.
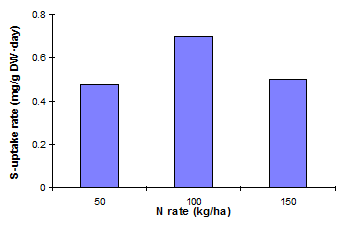
Fig. 15 Rates of S-uptake by mustard as influenced by rates of N
fertilizer (Source: Salim &
Rahmatullah, 1987)
Since both N and S are constituents of protein and involved in chlorophyll formation, absorption of N and S is interrelated (von Uexküll, 1986; Figure 15).
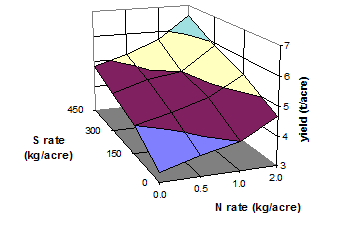
Fig. 16 Yield of garlic as influenced by rates of N and S fertilizer
(Source: Abd-Elfattah et al. 1990)
Addition of S to S-free N sources increases their effectiveness. Therefore, S is a good supplement of nitrogenous fertilizers (Figs 16 and 17). Recovery of N fertilizer in wheat was greater with added potassium sulfate (Abu-Zeid, 1992).
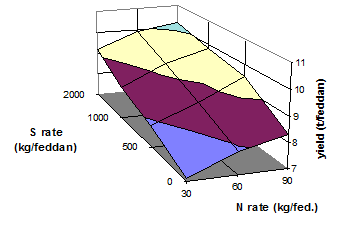
Fig. 17 Yield of squash as influenced by rates of N and S fertilizer
(Source: Hilal et al. 1990c)
3.5 Interactions between absorption of sulfur/chloride and other nutrients
K can improve uptake of S by crops (Figure 18) and S can increase absorption of K (chamomile El-Bahr, 1993; citrus Rabeh & Sweelam, 1990).
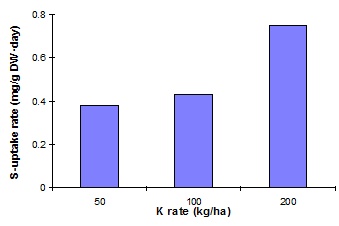
Fig. 18 Rates of S-uptake by mustard as influenced by rates of K
fertilizer (Source: Salim &
Rahmatullah, 1987)
The synergetic effects of N and K on sulfate absorption are also relevant for other nutrients (e.g. P Farrag et al. 1990; Table 13, Figs 19 and 20; Singh & Duhan, 1993). However when applied at high rates, K and P may decrease absorption of S by crops (e.g. corn Chowdhury et al. 1985). By contrast, application of sulfate may increase or decrease assimilation of other nutrients (Tables 14, 15, 16, and 17) with corresponding consequences for crop yield (Figures 21 and 22). This may be particularly true for vegetable crops: Abd-Elfattah et al. (1990) and Hilal et al. (1990c) encountered large synergetic effects of S and P on yields of garlic and squash, but not on wheat, fodder beet, and clover. Singh et al. (1995) found greater yield and higher bulb concentrations of N and P in garlic. K2SO4 and elemental S were regarded potentially useful to reduce uptake of radionuclides (Nisbet et al. 1994) such as cadmium, caesium and strontium by crops (e.g. potato Sparrow et al. 1994; rice Oh, 1988). This was, however, attributed to the potassium component in K2SO4 and the reducing effect of oxidation of elemental S in soil.
|
Table 13 Effects of P and S fertilizers on total N concentrations in bean seeds |
|||
|
P2O5 levels |
Sulfur levels |
||
|
|
0 g/pot |
|
15 g/pot |
|
|
(total N concentration in seeds) |
||
|
1.44 g/pot |
3.73 |
|
4.42 |
|
2.16 g/pot |
3.80 |
|
4.50 |
|
Source: Farrag et al. (1990) |
|||
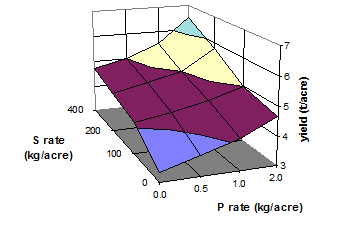 |
Fig. 19 Yield of garlic as influenced by rates of P and S fertilizer (Source: Abd-Elfattah et al. 1990)
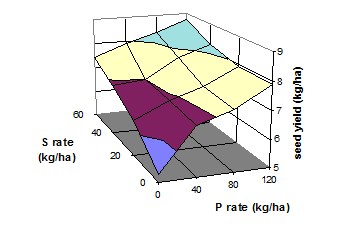
Fig. 20 Yield of squash as influenced by rates of P and S fertilizer
(Source: Hilal et al. 1990c)
|
Table 14 Effect of S fertilizer on nutrient uptake by peanuts |
|||
|
Nutrient |
Without S |
|
With S |
|
|
(content in plant; %) |
||
|
P |
0.52 |
|
0.59 |
|
K |
0.95 |
|
1.16 |
|
|
(content in plant; ppm) |
||
|
Fe |
580 |
|
838 |
|
Mn |
42.3 |
|
44.2 |
|
Zn |
35.6 |
|
52.3 |
|
Cu |
16.8 |
|
15.6 |
|
Source: Hilal et al. (1990b) |
|||
|
Table 15 Effect of S fertilizer on molybdenum uptake of broccoli |
|
|
S rate |
Mo (ppm) |
|
None |
5.09 |
|
50 ppm |
0.88 |
|
100 ppm |
0.50 |
|
Source: von Uexküll (1986) |
|
|
Table 16 Effect of S fertilizer on micronutrient uptake of lupines |
||||
|
S rate |
Fe |
Mn |
Zn |
Cu |
|
(kg/ha) |
(ppm) |
|||
|
0 |
1500 |
297 |
21.4 |
10.8 |
|
100 |
1610 |
376 |
31.6 |
16.8 |
|
200 |
1410 |
342 |
33.6 |
21.3 |
|
300 |
1360 |
280 |
42.7 |
26.7 |
|
Source: Hilal et al. (1990a) |
||||
|
Table 17 Effect of S fertilizer on micronutrient uptake of peas |
||||
|
S rate |
Fe |
Mn |
Zn |
Cu |
|
(kg/ha) |
(ppm) |
|||
|
0 |
1300 |
31.2 |
18 |
46.8 |
|
50 |
575 |
23.2 |
16 |
26.4 |
|
100 |
275 |
18.4 |
14 |
21.2 |
|
200 |
375 |
18.4 |
10 |
14.4 |
|
Source: Hilal et al. (1990d) |
||||
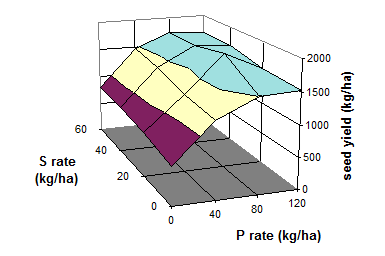
Fig. 21 Seed yield of soybean as influenced by S and P fertilization
(Source: Pasricha & Aulakh, 1990)
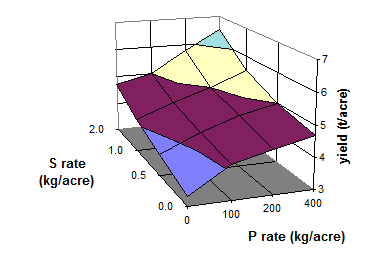
Fig. 22 Yield of garlic as influenced by S and P fertilization (Source: Abd-Elfattah et al. 1990)
It is understood that sulfate application increases absorption of SO4 when availability of soil S is lower than crop demand. S deficient soils are increasingly widespread, particularly in the tropics (e.g. Vaes, 1987; von Peter, 1981; Warman & Sampson, 1994a; Warman & Sampson, 1994b; Singh & Singh, 1984; Goraya et al. 1984). Deficiency of soil S is indicated when crops respond positively to application of S-containing fertilizers. If soil S is deficient, it is very likely that application of S increases absorption of other nutrients.
Not many authors have discussed the effect of chloride-containing fertilizers on absorption of other nutrients. von Uexküll & Sanders (1986) mentioned that higher rates of potassium chloride for oil palm significantly increased absorption of N and Ca (Table 18).
|
Table 18 Effect of potassium chloride on nutrient uptake of oil palm |
|||||
|
KCl rate (kg/tree) |
Leaf concentration (% in dry matter) |
||||
|
|
N |
P |
K |
Ca |
Cl |
|
0 |
2.56 |
0.165 |
0.890 |
0.678 |
0.146 |
|
0.5 |
2.67 |
0.169 |
0.859 |
0.725 |
0.421 |
|
1 |
2.60 |
0.167 |
0.869 |
0.755 |
0.545 |
|
Source: von Uexküll & Sanders (1986) |
|||||
Results from Jackson & McBride (1986) indicate a similar effect of KCl on absorption of Ca, particularly when compared with K2SO4 (Table 19). Callan & Westcott (1996) and Khamis et al. (1994) pointed out that K2SO4 suppressed uptake of Ca by cherry, pear, and peach trees. However, the potassium in KCl and K2SO4 may exert a competitive effect on absorption of Ca (e.g. in peanut Csinos & Gaines, 1986; mandarin Fahmy & Hassaballa, 1977; coffee Furlani et al. 1976).
|
Table 19 Effect of potassium fertilizers on petiole Ca concentration of potato |
|||
|
Potassium source |
Ca concentration at sampling date |
||
|
|
15 June |
28 June |
16 August |
|
0 |
1.26 |
1.35 |
1.50 |
|
KCl |
1.45 |
1.27 |
1.39 |
|
K2SO4 |
1.11 |
1.22 |
1.15 |
|
Source: Jackson & McBride (1986) |
|||
The exaggerating effects of Cl on absorption of Ca could be due to its influence on the water economy of plants since Ca is only passively transported in the transpiration stream. Consequently, greater water uptake by plants fed with KCl will increase absorption of Ca. However, this is only a weak argument in favor of the use of potassium chloride in the production of quality potato tubers (Chapter 4).
Potassium chloride was found to suppress P uptake but increase Mn uptake by cherry trees (Callan & Westcott, 1996). Increases in Mn uptake by KCl fertilization were also observed in wheat (Tu & Racz, 1994).
Malik et al. (1992) evaluated the effect of chloride on sulfate assimilation by Indian mustard: (1) without S application, Cl increased yields; (2) S uptake was decreased by Cl; (3) yields decreased with Cl rates when sulfate was applied.
Soils containing high salt concentrations can impede plant growth in several ways: (1) high osmotic pressure of the soil solution binds the soil water, renders it less available to plant roots and causes direct water stress in plants, (2) high salt concentrations of the soil solution can induce ionic imbalances in plants, (3) high concentrations can cause specific ion toxicity and physiological disorders in plants. Germination of seeds and growth of plants at all or different stages of development can be negatively affected by these factors. Specific ion toxicity is closely related to Cl toxicity and will be discussed in Chapter 4.
(1) Soluble salts depress the water potential in the soil solution by osmotic binding of soil water and thus restrict water uptake by plant roots. Osmoregulation (osmotic adjustment) is the process by which plants can adapt to water stress. Plants (a) absorb inorganic ions from the soil solution and (b) synthesize organic solutes which accumulate in the (a) cell vacuole and in the (b) cell cytoplasm. Important inorganic solutes absorbed are K+, Cl-, and NO3-. The most important organic osmotica is glycinebetaine (Hanson & Wyse, 1982). Uptake and synthesis of these species decreases the cell water potential, which promotes water uptake.
(2) The composition of ions in the soil solution in salt-affected soils is often unbalanced for plant requirements. Concentrations of K+ and Ca2+ are usually low whereas concentrations of ions such as B3+, Cl-, Na+, and Mg2+ can reach high levels. If absorbed in excessive quantities, these ions can induce physiological disorders or toxicity symptoms.
3.6.1 Salt tolerance at germination
Salt tolerance comprises the sum of above-mentioned effects on plant growth. Salt tolerance at germination (emergence) is measured as survival of plants (Maas, 1986b). Many plants are more salt tolerant at germination and become increasingly tolerant during later growth stages. A comparison of salt tolerance of crops at the germination stage is shown in Table 20.
|
Table 20 Salt tolerance of crops at germination as measured by the electrical conductivity of the soil extract at which 50 percent of seedlings emerged compared with a non-saline control |
|
|
Crop |
50% Emergence at electrical conductivity (dS/m) |
|
Corn |
21-24 a |
|
Barley |
16-24 |
|
Rice |
18 |
|
Cowpea |
16 |
|
Wheat |
15-16 |
|
Cotton |
16 |
|
Beetroot |
13.8 |
|
Sorghum |
13 |
|
Common cabbage |
13 |
|
Alfalfa |
8-13 |
|
Safflower |
12 |
|
Sugarbeet |
6-12 |
|
Lettuce |
11 |
|
Bean |
8 |
|
Tomato |
7.6 |
|
Onion |
5.6-7.5 |
|
a greater numbers indicate better salt tolerance Source: Maas (1986b) |
|
Germination of many crops can be poor under saline soil conditions (e.g. wheat Petrov-Spiridonov, 1991) but especially vegetables are sensitive to even low salt concentrations. Under non-saline conditions, basal applications of fertilizers with a high salt index (Table 6) should be avoided. Experiments with onions, carrots and bush beans at the Experimental Station Kamperhof in Germany (Versuchsanstalt Kamperhof, 1997: personal communication) show the favorable effects of potassium sulfate on germination of these vegetables: germination rate was higher with K2SO4 than without potassium fertilizer (except bush bean). KCl always depressed germination in vegetables (Figure 23).
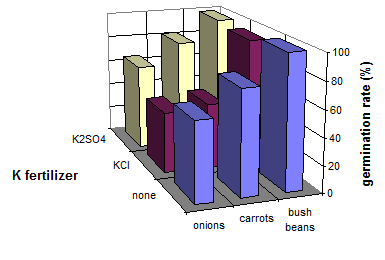
Fig. 23 Germination in three vegetables as influenced by K fertilization
(Source: Versuchsanstalt Kamperhof,
1997: personal communication)
3.6.2 Salt tolerance after germination
Salt-tolerance of crops after emergence may differ from their tolerance at germination. Maas (1986b) estimated salt-tolerance for a large number of crops. A selection is presented in Table 21. Particularly vegetables and fruit trees are sensitive to high ion concentrations of the soil solution. Experiments with onions, carrots and bush beans at the Kamperhof in Germany (Versuchsanstalt Kamperhof, 1997: personal communication) show the favorable effects of potassium sulfate on vegetable growth in the post-germination phase: height of seedlings was greater with K2SO4 than without potassium fertilizer (except bush bean). KCl always depressed growth of seedlings. (Figure 24). Damages are exaggerated at certain environmental factors such as hot, dry conditions.
Salt-induced damage of woody crops is usually caused by high concentrations of Cl and Na in leaves. Different cultivars or rootstocks absorb those ions at markedly different rates. However, varieties that restrict the uptake of Cl and Na ions may be negatively affected by osmotic effects of the soil solution. Crops irrigated by overhead sprinkler systems may be particularly affected by saline water. This depends on leaf characteristics and rate of foliar absorption of crops (Maas, 1986b).
|
Table 21 Rating of salt tolerance of crops after germination |
||||
|
tolerant |
||||
|
barley |
cotton |
sugarbeet |
asparagus |
date palm |
|
medium tolerant |
||||
|
cowpea |
safflower |
sorghum |
soybean |
wheat |
|
pineapple |
oats |
rape |
artichoke |
zucchini |
|
medium sensitive |
||||
|
corn |
peanut |
sugarcane |
alfalfa |
cabbage |
|
eggplant |
lettuce |
chili a |
potato |
radish |
|
spinach |
grape |
sunflower |
cauliflower |
cucumber |
|
sensitive |
||||
|
bean |
rice |
beetroot |
carrot |
onion |
|
strawberry |
apple |
avocado |
cherry |
mango |
|
orange |
peach |
pear |
plum |
okra |
|
a from the authors personal experience chili should be rated sensitive Source: Maas (1986b) |
||||
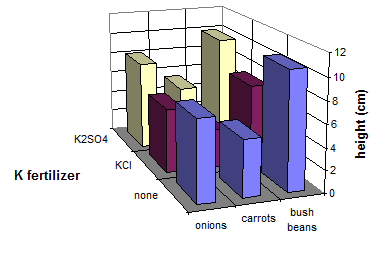 |
Fig. 24 Height of seedlings of three vegetables as influenced by K fertilization (Source: Versuchsanstalt Kamperhof, 1997: personal communication)
4 Sulfur and Chloride in Plants
4.1 Translocation of sulfate and chloride
After absorption by roots sulfate is mainly transported with the transpiration stream in upward (akropetal) direction. Translocation of SO42- against the transpiration stream in a downward (basipetal) direction is only limited. Plants deficient in S absorb sulfate at a high rate. This S is initially transported to the older parts. After their demand has been met, S is translocated to the younger plant parts (Mengel, 1991). However, the S of the older leaves does not contribute to the S supply of younger tissues. Chloride is highly mobile in plants and can easily move in akropetal and basipetal direction. It accumulates mainly in the vegetative plant parts.
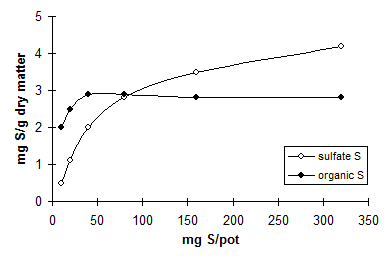
Fig. 25 Effect of S supply on inorganic (SO42-) and
organic S in leaves of sunflower (Source: Mengel,
1991)
S in plants occurs in organic and inorganic form. There is no clear relationship between concentrations of organic and inorganic S. At low S supply the greater part of absorbed S is incorporated into organic compounds. With increasing supply and absorption the ratio organic:inorganic S in plants decreases (Figure 25). Once the demand for organic S has been satisfied the content of inorganic S increases. Sulfate serves as a reservoir for further assimilation into organic compounds. Therefore, determination of sulfate-S can show whether a plant is sufficiently supplied with S (von Uexküll, 1986): if inorganic sulfate is present, the S supply meets demand of the plant.
Assimilation refers to the incorporation of ions into organic molecules. Assimilation of sulfate absorbed by plants (S6+) requires (1) its activation and subsequently (2) its reduction to the S2- of amino acids. Reduction of sulfate-S is carried out by enzymes located in the membrane of chloroplasts. Therefore, SO42- must be translocated to the photosynthetic active plant parts. The reactions involved require energy, especially in the form of ATP. Hence, assimilation of sulfur depends on photosynthesis and is mainly carried out during the light period.
4.2.1 Sulfate activation and reduction
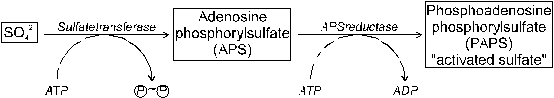
Fig. 26 Reduction of absorbed sulfate in plants: (1) activation
(Source: Amberger, 1979)
Activation of sulfate consumes energy from the reaction of ATP to ADP + Pi. The first step of S reduction is the activation of SO42- yielding adenosine phosphorylsulfate (APS). This reaction is catalyzed by the enzyme Sulfatetransferase. APSreductase transfers APS to the activated sulfate phosphoadenosine phosphorylsulfate (PAPS, Figure 26).
Following its activation, sulfate is reduced to carrier-bound sulfite (carrierSSO3H). In this reaction, phosphoadenosine phosphate (PAP) is split off from PAPS by Sulfatetransferase. To be incorporated into organic forms sulfite is reduced to sulfide (carrierSSH). The required electrons for this reaction are supplied by Ferredoxin from the photosynthesis (Figure 27). Ferredoxins are FeS proteins and the first stable redox compounds of the photosynthetic electron chain (Figure 28). In addition to the S contained in the cysteine and methionine units of their protein chain, they contain additional S and Fe atoms. Occurring in the chloroplasts, ferredoxin plays an important role in the redox system of photosynthesis. As a strong reducing agent, it is ultimately responsible for the reduction of CO2 and other compounds (e.g. SO4, N2, glutamate).
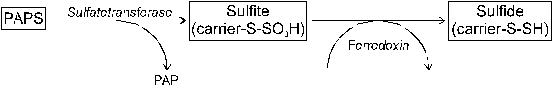
Fig. 27 Reduction of absorbed sulfate in plants: (2) reduction (Source:
Amberger, 1979)
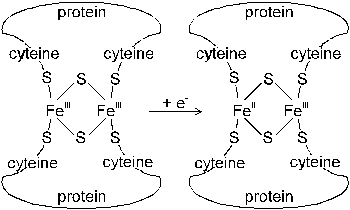
Fig. 28 The ferredoxin redox system (Source: Mengel & Kirkby, 1987)
4.2.2 Incorporation of S into organic forms
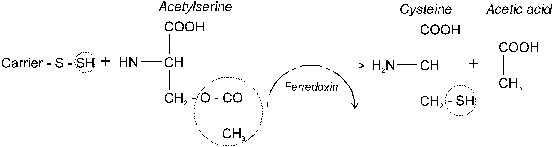
Fig. 29 Formation of cysteine from absorbed S (Source: Amberger, 1979)
Usually, reduced S is rapidly incorporated into organic molecules. The SH group of the carrier complex is transferred to the activated amino acid acetylserine. Ferredoxin serves as an electron source for the formation of cysteine (Figure 29). Cysteine is the first stable organic S form and precursor of other amino acids such as cystine and methionine, and several S-containing biochemical compounds.
4.3 S-containing organic compounds
S-containing organic compounds in plants can be divided into chemical categories according to the specific type of S bond they contain. For agronomic purposes, some of these molecules can be classified according to specific substance categories.
In organic molecules S occurs (1) in sulfide bonds (RSH, R1SR2), (2) in disulfide bonds (R1SSR2), (3) as rhodanide (RN==C==S), and (4) in thiazole rings of heterocyclic S compounds (Mengel, 1991).
4.3.1 Sulfide bond (RSH, R1SR2)
Fig. 30 Sulfide bonds in amino acids
Sulfide bonds occur in S-containing amino acids (Figure 30). They account for the bulk of the S in plants (90 %) unless they contain leek or mustard oils.
The highly reactive SH groups are essential for the formation of disulfide bonds in polymerization of polypeptides and proteins. At the same time, they also have important metabolic functions in plants, e.g. enzymatic reactions. Many enzymes contain SH groups. One outstanding example is coenzyme A, which reacts with organic acids to form acetyl-CoA (Figure 31). This activated acid plays a significant role in fatty acid and lipid metabolism of plants. The methyl group of methionine is involved in biosynthesis of lignin, pectin, chlorophyll, and flavonoids.
Fig. 31 Formation of acetyl-CoA from coenzyme A (Source: Mengel & Kirkby, 1987)
Volatile S compounds are of great significance in agricultural production since many plant species contain at least small amounts of them. Sulfide bonds can be found in leek oils (e.g. dimethylsulfide, dimethyldisulfide, diallyldisulfide, alliine) as characteristic constituents of the Alliaceae (e.g. leek, onion, garlic). Important constituents of the Cruciferae (e.g. radish, horseradish, cress, brassicas) are mustard oils (e.g. methylisothiocyanate, sinigrine, benzylisothiocyanate, erucine). Besides the S present in amino acids, those plant species contain significant amounts of S in such compounds.
4.3.2 Disulfide bond (R1SSR2)
One major function of S in plants is the formation of disulfide bonds in proteins or polypeptide chains. Disulfide bonds are formed from two SH groups:
2 RSH Ö R1SSR2 + 2 H
Disulfide bridging fulfills two fundamental roles in plant metabolism. (1) Disulfide bonds serve as linkages between the polypeptide chains in a protein molecule. Therefore, they contribute to conformation and polymerization of polypeptides and proteins. (2) The (a) cysteine, (b) glutathione, and (c) lipoic acid systems are important redox systems in plants.
(1) Cysteine and methionine are the most important S-containing amino acids in plants. Both occur as free acids and act as building blocks of proteins. S-containing proteins are synthesized by formation of disulfide bonds between polypeptide chains.
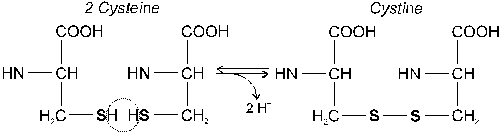
Fig. 32 Synthesis of a dipeptide through formation of a disulfide bond:
(1) the cysteine redox system (Source: Amberger,
1979)
(2) Redox systems are essential for plant metabolism. (a) The dipeptide cystine and two protons are synthesized from two cysteine molecules (Figure 32).
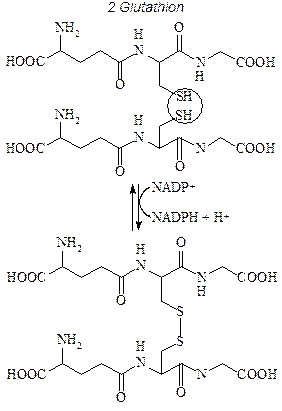
Fig. 33 Synthesis of a dipeptide through formation of a disulfide bond:
(2) the glutathione redox system (Source: Mengel
& Kirkby, 1987)
(b) The glutathione system is analogous to the cysteine system (Figure 33). However, it plays a more significant role in metabolism due to its higher solubility.
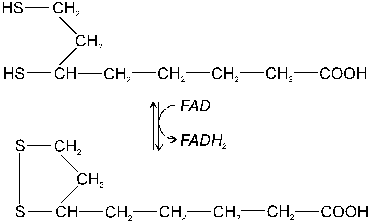
Fig. 34 The lipoic acid redox system. The disulfide bond does not connect
amino acid chains (Source: Mengel &
Kirkby, 1987)
(c) The coenzyme lipoic acid contains two SH groups in its reduced form. Unlike the cysteine and glutathione redox systems, the disulfide bridge does not connect two amino acid chains (Figure 34). Leek oils such as dimethylsulfide and diallyldisulfide contain such disulfide bonds.
As shown in the figures, all reactions are reversible. Depending on the redox conditions the systems can function as electron donators or acceptors. Under reducing conditions the equilibrium is shifted towards cysteine, and glutathione and lipoic acid in their reduced sulfide forms. This consumes two protons. Under oxidizing conditions cystine is formed, and glutathione and lipoic acid are polymerized to their disulfide forms. Two protons are released.
Important rhodanide-type organic compounds are isothiocyanates such as mustard oils (e.g. methylisothiocyanate, benzylisothiocyanate, and erucine). In plants, they do not occur as mustard oils per se but as mustard-oil glucosides or glucosinolates. Glucosinolates are synthesized from amino acids in three steps (Figure 35). They can be hydrolyzed upon utilization or enzymatic decomposition to isothiocyanates (e.g. mustard oil), glucose and sulfate (Figure 36). The radical R in the formulas varies for different glucosinolates and isothiocyanates.
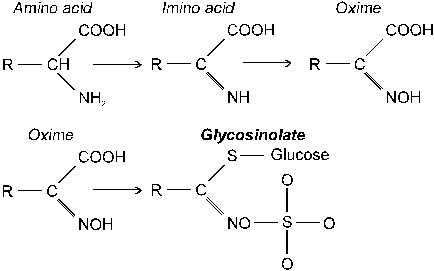
Fig. 35 General synthesis of glucosinolates from amino acids
(Source: Mengel & Kirkby,
1987)
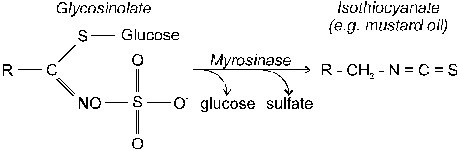
Fig. 36 General synthesis of isothiocyanates from glucosinolates (Source: Mengel & Kirkby, 1987)
4.3.4 Heterocyclic S compounds (thiazole ring)
In heterocyclic S-containing molecules, the S is present in thiazole rings. Thiamin (vitamin B1) and Biotin (vitamin H) are two important examples (Figure 37). In plants, both occur largely in the grains of cereals and legumes as free vitamins that are involved in metabolism.
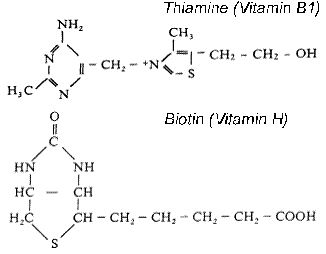
Fig. 37 Heterocyclic S compounds in plants
4.3.5 Classification of S-containing organic compounds
Division of S-containing organic compounds into S-bond and substance categories is not the same. For example, dimethylsulfide contains a sulfide bond (R1SR2) and dimethyldisulfide a disulfide bond (R1SSR2) although both compounds belong to the leek oils. Therefore, for agronomic purposes the organic S-compounds should preferably be separated into substance categories (Table 22).
4.4 Effects of S supply on organic compounds and quality of crops
4.4.1 Effects on photosynthesis
Photosynthesis (light reaction) is the absorption of electromagnetic radiation and its conversion into chemical forms of energy. CO2 assimilation (dark reaction) is the conversion process of inorganic C into organic forms requiring energy provided by photosynthesis (Figure 38). The chain of enzymatic reactions in which CO2 is fixed into carbohydrate is termed Calvin cycle. Inside the chloroplast, the carbon from CO2 reacts with molecules of cycling carbon sugar phosphates to form dihydroxyacetone phosphate (DHAP), a precursor of other carbohydrates (i.e. photosynthates) synthesized in the cytosol outside the chloroplast. Those include a large number of polysaccharides, fatty acids and amino acids: sucrose as a main end product of photosynthesis in higher plants (table sugar) is synthesized by sucrose synthetase from phosphorylated forms of glucose and fructose.
|
Table 22 Important S-containing organic compounds in plants |
||
|
Name |
Chemical formula |
Description and function |
Ferredoxin |
redox component of the photosynthetic electron chain, electron donator for synthesis of cysteine, responsible for the reduction of CO2 and other compounds |
|
|
|
||
|
Amino acids |
building blocks of protein |
|
|
Cysteine |
(see figure 30) |
precursor of amino acids and other S compounds, polymerization of polypeptide chains, redox system |
|
Methionine |
biosynthesis of lignin, pectin, chlorophyll and flavonoids |
|
|
|
|
|
|
Coenzyme A Acetyl Co A |
redox system, reacts with organic acids component of protein and lipid metabolism, photosynthesis (see figure 31) |
|
|
|
|
|
Glutathione |
redox system, polymerization of polypeptide chains (see figure 33) |
|
|
|
|
|
Lipoic acid |
redox system (see figure 34) |
|
|
|
|
|
Thiamin, biotin |
vitamins, metabolism (see figure 37) |
|
|
|
|
|
|
Leek oils |
mainly in Alliaceae (leek, onion, garlic, etc.) but also in peppermint, pineapple, etc. In plants they occur as odorless, oxidized compounds which can be reduced by enzymatic reactions. |
|
|
Dimethylsulfide |
CH3-S-CH3 |
in peppermint oil |
|
Dimethyldisulfide |
CH3-S-S-CH3 |
in peppermint oil |
|
Diallyldisulfide |
CH2=CH-CH2-S-S-CH2-CH=CH2 |
main component of garlic oil |
|
Alliine |
CH2=CH-CH2-S(O)-CH2-CH(NH2) |
in garlic |
|
|
|
|
|
Mustard oils |
mainly in Cruciferae (mustard, radish, horseradish, cress, brassicas, etc.). In plants they occur as glucosides or glucosinolates which can be decomposed to mustard oils by enzymes. |
|
|
Methylisothiocyanate |
CH3-N=C=S |
odor of horseradish |
|
Sinigrine |
CH2=CH-CH2-C-S-C6H11O5 + H2O = N-O-SO3-K |
in many Cruciferae, e.g. black mustard |
|
Benzylisothiocyanate |
C6H5-CH2-N=C=S |
odor of cress, in Raphanus sativus |
|
Erucine |
CH3-S-[CH2]4-N=C=S |
odor of radish, in Eruca sativa |
Sucrose is highly mobile in plants and can be transported to other plant parts to be reformed to glucose. Starch as a major polysaccharide made up of glucose residues is formed by starch synthetase in the chloroplast as well as in the cytosol. As a major storage carbohydrate of plants, it accumulates in storage organs where it is formed from carbohydrates translocated from leaves. Acetyl-CoA is formed in chloroplasts and mitochondria from carbon sugar phosphates from the Calvin cycle. Fatty acids are synthesized from acetyl-CoA. Those comprise unsaturated and saturated fatty acids. Unsaturated fatty acids are characterized by a number (usually 1 to 3) of double bonds and lower melting points. A large number of enzymes are involved in the synthesis of unsaturated fatty acids which are required for plant metabolism. Saturated fatty acids are formed by fatty acid synthetase. Oils and fats are stored as glycerol-bound fatty acids (Figure 38). Important fatty acids in plant oils are oleic and linoleic acid. Liquid oils contain more unsaturated fatty acids whereas solid fats contain mainly saturated fatty acids.
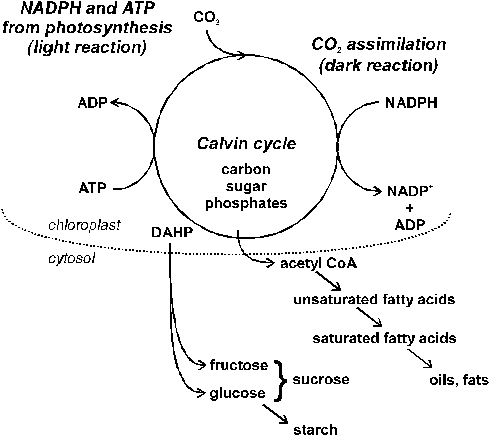
Fig. 38 CO2 assimilation, and formation of polysaccharides and
fatty acids
Sulfur is connected with photosynthesis of plants as follows: (1) assimilation of sulfur depends on photosynthesis of plants since reduction of absorbed sulfate is carried out by enzymes in the membrane of chloroplasts. (2) Ferredoxin and Acetyl-CoA contain S and play a significant role in the reduction of CO2 and production of organic compounds. Although the chlorophyll of plants does not contain sulfur, S deficiency affects chloroplast formation and may even lead to decomposition of chloroplasts (von Uexküll, 1986). Deficiency in S-containing amino acids probably interferes with synthesis of proteins necessary for the formation of chloroplasts (Amberger, 1979).
In contrast to N deficiency, chlorotic symptoms (yellowing) appear first in the younger, most recently formed leaves. The S in older plant tissues does not contribute to the S supply of younger leaves, which consequently depends mainly on root uptake. Many plant species develop a reddish or purplish tint, especially on the underside of the leaves. Chlorosis of leaves is followed by inhibited shoot growth expressed as thin and shortened internodes. A prominent example of S deficiency is the yellow disease or tea yellows in tea (Mengel, 1991). S deficiency is particularly serious in the Cruciferae: similar to S-deficiency symptoms in tea, plants remain small and stunted. Lateral extension of leaves is restricted. Therefore, they remain narrow and are often curled upwards (von Uexküll, 1986).
4.4.2 Effects on amino acids and proteins
The total S content in plant tissues is in the range of 0.2 to 0.5 percent in the dry matter. Except plant species which contain large amounts of leek and mustard oils the bulk of organic S in plant tissues consists of amino-acid and protein S. A high S requirement is characteristic of protein-rich crops like legumes. Because the S-containing amino acids cysteine and methionine are essential building blocks of proteins, S deficiency results in a general reduction of protein synthesis (von Uexküll, 1986). Non-S-containing soluble amino acids (e.g. asparagine, glutamine, and arginine; soluble organic N in figure 39) and nitrate-N accumulate in plant tissues. This is associated with low concentrations of sugars resulting from the poor photosynthetic activity of S-deficient plants. Consequently, the growth rate of S-deficient plants is reduced (fresh weight in figure 39).
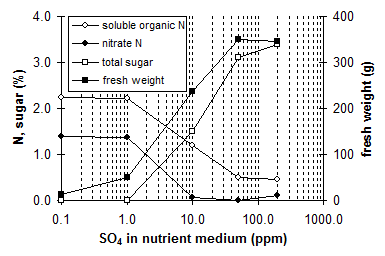
Fig. 39 Effects of S supply on nitrogen and sulfur compounds in leaves, and
on fresh weight of cotton (Source: Mengel
& Kirkby, 1987)
Under conditions of S deficiency, synthesis of S-containing amino acids and protein is depressed. Therefore, application of S is frequently associated with increases in their content in crops. Llanos Company (1984) mentioned the positive effect of S on the synthesis of S-containing amino acids in tobacco. K2SO4 produced significantly higher protein content in wheat than KCl (Baksh et al, 1986). K2SO4 improved protein levels in rape (Hocking et al. 1996) and protein content rice (Trivedi & Verma, 1996).
Increases in amino acid and protein content by S fertilization are particularly true for legumes (Tables 23, 24 and 25). This may also be related to the effect of sulfate on nodule bacteria, improving fixation of atmospheric nitrogen and thereby increasing protein content (von Peter, 1981; Collins & Duke, 1981). This has usually significant effects on the yield of legumes (Singh & Duhan, 1993; Naik et al. 1993; El-Desoky et al. 1993; Sarkar et al. 1991; El-Fouly et al. 1989; Karwasra & Raj, 1984). However, excessive S levels may depress protein yields in crops (Figure 40) probably through metabolic unbalances (Farrag et al. 1990). S fertilization may not affect the S content of the reproductive plant organs (grains and seeds) as much as the S content of the non-reproductive organs (stems and leaves). When the S demand for synthesis of organic compounds in the seeds is satisfied, excessive amounts of absorbed S remain in the non-reproductive plant parts (Mengel, 1991).
|
Table 23 Effect of KCl and K2SO4 on amino-acid content of green tea seedlings |
|||
|
|
KCl |
|
K2SO4 |
|
91 |
|
117 |
|
|
Source: Wu & Ruan (1994) |
|||
|
Table 24 Effect of KCl and K2SO4 on protein content of Lucerne and wheat |
|||
|
Crop |
Protein content (%) |
||
|
|
KCl |
|
K2SO4 |
|
Lucerne |
12.5 |
|
14.4 |
|
wheat |
13.6 |
|
17.5 |
|
Source: Saurat & Boulay (1985) |
|||
|
Table 25 Effect of S application on protein content of grains |
||||
|
Protein content (%) |
% increase |
|||
|
|
without S |
|
with S |
|
|
Rice |
|
|||
|
Maize |
8.8 |
|
1 |
|
|
Chickpea |
20.2 |
|
23.5 |
16 |
|
Greengram |
24.4 |
|
26.3 |
8 |
|
Blackgram |
22.5 |
|
24.0 |
7 |
|
Pigeonpea |
23.5 |
|
25.0 |
6 |
|
Lentil |
26.0 |
|
27.5 |
6 |
|
Soybean |
28.1 |
|
31.9 |
14 |
|
Groundnut |
27.5 |
|
30.4 |
11 |
|
Sunflower |
13.9 |
|
16.6 |
19 |
|
Rapeseed mustard |
22.9 |
|
36.8 |
34 |
|
Yellow mustard |
23.3 |
|
28.1 |
21 |
|
Source: Tandon (1991) |
||||
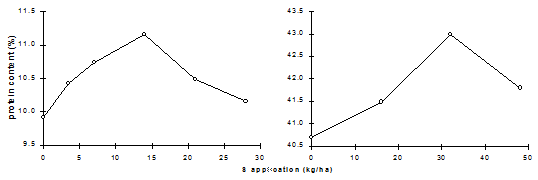
Fig. 40 Effect of K2SO4 application on protein
content of (left) wheat and (right) soybean (Sources: Germida, 1990; Chaiwanakupt
et al. 1987)
The different S-containing amino acids may not be uniformly affected by S application. For quality aspects of protein-rich crops, it is important whether the content of the free amino acids cysteine and methionine, or contents of the synthesized polypeptides and proteins can be increased by application of S. However, experimental results are inconsistent in this respect (Tables 26 and 27, Figures 41 and 42).
|
Table 26 Effect of S on amino-acid content of crops |
|||
|
Crop |
Increase (%) |
||
|
|
Cysteine |
Methionine |
Cystine |
|
Rice |
167 |
85 |
110 |
|
Soybean |
58 |
117 |
52 |
|
Rapeseed mustard |
20 |
9 |
16 |
|
Source: Tandon (1991) |
|||
|
Table 27 Effect of S on amino-acid and protein content of rice |
||
|
|
Methionine (%) |
Crude protein (%) |
|
without S |
0.27 |
7.81 |
|
with S |
0.38 |
8.62 |
|
Source: Morris (1986) |
||
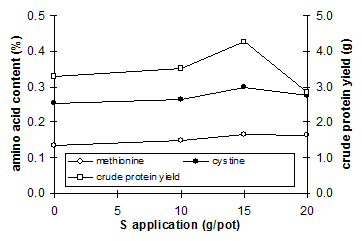
Fig. 41 Effect of S on amino acid and protein content of bean seeds (Source:
Farrag et al. 1990
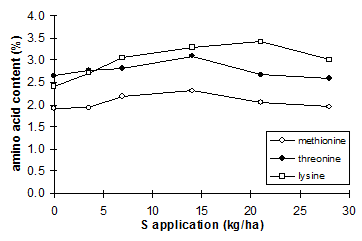
Fig. 42 Effect of K2SO4 application on protein
content of wheat (Source: Germida, 1990)
4.4.3 Effects on lipids (oils and fats)
S is an essential building block of important oil compounds. In plant species capable of synthesizing such oils, they are the primary storage forms of S. Therefore, the content of oil in plants is closely related to S supply. Fertilization usually results in increases in the oil content and consequently yields of plant seeds. This is particularly true for the Cruciferae, which produce appreciable amounts of mustard oils (Table 27).
|
Table 27 Effect of S on oil content in seeds of crops |
|
|
Crop |
Increase in oil content (%) |
|
sesame |
2.9 |
|
groundnut |
11.3 |
|
linseed |
6 |
|
mustard |
9.6 |
|
sunflower |
3.8 |
|
safflower |
2 |
|
soybean |
9.2 |
|
Source: Tandon (1991) |
|
Onions are known for their high sulfur requirement (Tandon & Kemmler, 1986). Compared with KCl, application of K2SO4 increased contents of glucosinolates and oils in S-deficient rape in Australia (Hocking et al. 1996). The high S needs of peanut are indicated by improved plant growth (Nabi et al. 1990) and greater pod, seed and oil yields (Siddaramappa et al. 1993). The proportion of phospholipids and free fatty acids increased as well as the proportion of unsaturated fatty acid (Sukhvinder et al. 1994). Thirumalaisamy et al. (1986) noticed higher carbohydrate, protein and oil contents in peanut seeds.
Besides increasing its contents, S may also improve the quality of oils in crops (Table 28).
|
Table 28 Effect of S on oil content and oil quality of mustard |
|||
|
S rate |
Oil content (%) |
Acid value of oil |
Saponification value of oil |
|
0 |
46.40 |
0.75 |
81.90 |
|
30 |
47.34 |
0.99 |
85.27 |
|
60 |
49.03 |
1.16 |
99.10 |
|
90 |
48.24 |
0.87 |
121.54 |
|
Source: Salim & Rahmatullah (1987) |
|||
This is particularly interesting for production of pharmaceutical plants. K2SO4 improved oil yields of Silybum marianum (Omer et al. 1996) and fat content of Glossostemon bruguiri (El-Gengaihi et al. 1995) in Egypt. In contrast to synthesis of amino acids and proteins in plants, increased S supply can enhance oil contents even beyond the point of maximum plant yield (Mengel & Kirkby, 1987).
Sulfur deficiency is believed to affect carbohydrate metabolism in sugarcane (Rahman et al. 1986). Application of S significantly increased sugar yields at three locations in Bangladesh (Table 29) and juice purity in India (Tandon, 1991).
|
Table 29 Effect of S on sugar yield of sugarcane |
||
|
Location |
Sugar yield (t/ha) |
|
|
|
without S |
with S |
|
SRTI |
8.63 |
8.75 |
|
RJSM |
5.66 |
6.35 |
|
STSM |
2.85 |
3.45 |
|
Source: Rahman et al. (1986) |
||
Compared with KCl, K2SO4 improved contents of sugars in grapes and alcohol content in wine (Saurat & Boulay, 1985; Tandon & Kemmler, 1986). In pineapple, K2SO4 frequently increases the sugar content and decreases acidity (Marchal et al. 1981; Anonymous, 1979).
The effects of S on starch content of crops have been extensively studied in potatoes (Zehler et al, 1981). Generally, tubers and stems of potato have relatively high S contents (up to 0.3 and 0.5 percent). Compared with KCl, K2SO4 usually produces higher starch contents, and a higher proportion of coarse starch granules in potatoes. Quality parameters such as ascorbic acid, amino acid and protein content, specific gravity and viscosity, and post-harvest quality are commonly improved by S. Beringer et al. (1990) attributed such effects to higher translocation of assimilates into tubers, caused by a greater sink strength of tubers compared with shoots (Figure 43).
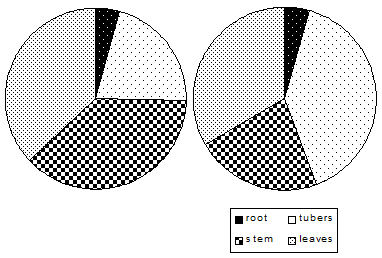
Fig. 43 Effect of (left) KCl and (right) K2SO4 on
distribution of 14CO2 in potato (Source: Haeder, 1976)
Greater sink strength of tubers reduces shoot growth but increases yields in potato. Sugars are the building blocks of starch. Since K and S improve synthesis of starch from sugars, they lower contents of sugar in tubers. This improves the quality (low tendency to undesirable browning, reduced formation of volatile compounds in cooking) of starch, table and industrial food potatoes (Orlovius, 1996). At eight locations in Germany, K2SO4 improved tuber starch contents by an average of 0.5 to 1.0 percent. However, many such effects are due to detrimental effects of chloride on translocation of assimilates into tubers (see later). Similar to the effects on potato, application of S increased starch contents in tropical root crops such as cassava (Tandon, 1991) or taro (Ni, 1994).
There is indication that S fertilization also improves contents of starch in grain crops such as rice: Particularly in Asia, many authors have pointed to the benefits of sulfur to improve rice yields, which are, to a certain extent, equivalent to starch and protein yields (e.g. Morris, 1986; Ismunadji, 1991a; Biswas & Tewatia, 1991; Mamaril et al. 1991; Blair, 1986; Liu, 1986; Blair et al. 1991; Islam, 1986; Liu et al. 1993; Vaes, 1987; Shah, 1987; Bhuiyan, 1991). Despite the potentially harmful effects of reduction of sulfate to sulfide in flooded soils (Chapter 2.2.4), some authors have tested potassium sulfate as a S source for rice. There may be no negative impacts of reduction of sulfate when potassium sulfate is surface applied. Samosir (1991) suggested surface-applied potassium sulfate for controlling S-deficiency of rice in Indonesia. Trivedi & Verma (1996) found higher carbohydrate contents in rice fertilized with K2SO4 compared with KCl. Consumption of K2SO4 for rice is increasing in Japan (von Peter, 1981). Gurmani et al. (1984) tested potassium fertilizers for rice production in Pakistan and found significant yield increases with K2SO4 in 25 trials (Table 30). In India, application of ammonium sulfate to 24 soils increased rice yield by an average of 11 percent (Table 31).
|
Table 30 Effect of KCl and K2SO4 on rice yield and value/cost ratio in Pakistan |
||
|
Potassium fertilizer |
Yield (t/ha) |
Value/cost ratio |
|
none |
4.91 |
|
|
KCl |
5.52 |
9.34 |
|
K2SO4 |
6.11 |
12.35 |
|
Source: Gurmani et al. (1984) |
||
|
Table 31 Effect of KCl and K2SO4 on rice yield in India |
|
|
Potassium fertilizer |
Yield (g/pot) |
|
KCl |
42.8 |
|
K2SO4 |
47.6 |
|
Source: Tiwari et al. (1983) |
|
Chowdhury & Majumder (1994) found significant yield increases in rice by S application irrespective of source (elemental S, gypsum, K2SO4 or magnesium sulfate). Potassium sulfate and ammonium sulfate were similarly effective in increasing rice yields in Pakistan (Rashid et al. 1992). Ismunadji (1991b) pointed out that potassium sulfate is equally effective as other S sources in Indonesia. Yamauchi (1989) controlled bronzing of rice by application of K2SO4. In contrast, chloride increased the severity of bronzing and let to deterioration of growth.
4.4.6 Interactions between S and other nutrients
All the nutrients essential for plant life interact continuously in the plant cell. In addition to the effects of nutrient interactions on crop yields already presented in Chapter 3, they also modify contents and relations of organic compounds.
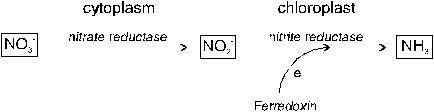
Fig. 44 Nitrate and nitrite reduction in plants
Since both nitrogen and sulfur are constituents of protein and involved in chlorophyll formation, their supply is closely connected with biosynthesis of organic compounds in plants. Nitrogen is present in all amino acids, proteins and coenzymes, and S in some of them. When S supply is deficient, synthesis of S-containing organic compounds is inhibited. Nitrate is often the major source of N, particularly for the dicotyledonous plants. NO3 is absorbed rapidly and even against concentration gradients. This can result in accumulation of nitrate-nitrogen that has been absorbed but cannot be incorporated into organic substances (Hilal et al. 1990a). Lack of S supply can directly affect photosynthesis in several ways. (1) S deficiency has a negative effect on chloroplast formation (see earlier this Chapter). (2) The S-rich protein ferredoxin is involved in reduction of absorbed nitrate (Figure 44).
(3) If S is deficient, non-S-containing soluble amino acids accumulate in plant tissues (Figure 39). These amino acids may depress the activity of nitrate reductase (Srivastava, 1980). For these reasons, S-containing fertilizers may have a significant impact on promoting nitrate reduction (Table 32), increasing nitrogen use efficiency (Table 33) and reducing nitrate contents particularly in leafy vegetables (Figure 45). He et al. (1994) showed that K2SO4 reduced leaf nitrate but increased amino-acid content of cabbage. Both effects are probably interrelated: greater nitrate reduction provides more N for synthesis of amino acids.
|
Table 32 Effect of KCl and K2SO4 on nitrate reduction in tea seedlings |
|||
|
|
Fertilizer |
||
|
|
NP |
KCl |
K2SO4 |
|
Nitrate reduction (%) |
100 |
79 |
120 |
|
Source: Wu & Ruan (1994) |
|||
|
Table 33 Effect of S on nitrate reductase activity and nitrogen use efficiency in sugarcane |
||
|
S application rate (kg S/ha) |
Nitrate reductase activity (n mol N2/g f.w/hr) |
Nitrogen use efficiency (dm, g/g N/m2) |
|
0 |
1652 |
2.17 |
|
40 |
1775 |
2.23 |
|
80 |
1989 |
3.02 |
|
120 |
2020 |
2.54 |
|
160 |
1805 |
2.67 |
|
Source: Shanmugam (1995) |
||
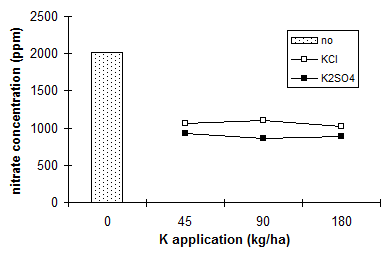
Fig. 45 Effect of KCl and K2SO4 on nitrate concentration of mustard (Source: Ni, 1995)
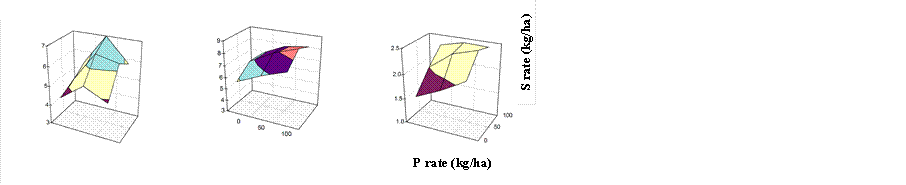
Fig. 46 Effect of S and P fertilization on (left) protein, (middle) sugar,
and (right) fat content of sorghum (Source: VON UEXKÜLL, 1986)
4.5 Functions of chloride in plants
Major functions of Cl- in plants are: (1) it is a counter-ion for cation transport, (2) it acts as an osmotic solute contributing to cell hydration and turgor, (3) it regulates stomatal activity, (4) it has biochemical functions in metabolic processes in the plant and (5) it may suppress disease infections (Maas, 1986a; Halstead et al. 1991).
(1) As a counter-ion, Cl- maintains electrical charge balance for the uptake and translocation of nutrient cations by plants. The effect of chloride on uptake of nutrients has been outlined in Chapter 3.
(2) By lowering the water potential of plant tissues, Cl- increases the ability of plants to extract soil water and improves their tolerance to saline conditions. Sugar beet as a chenopodiaceae is an economically important crop requiring Cl- for increasing leaf succulence. Both inorganic and organic solutes affect the osmotic potential of plant cells. The most important inorganic osmotic solutes, which can predominately be found in the vacuoles, are K+, Na+ and Cl-. Organic osmotica such as glycerol, amino acids and sucrose are present in the cytoplasm of plant cells. However, the ability of Cl- to move rapidly across cell membranes makes it particularly important as an osmoticum in plants. There is indication that Cl- is sequestered in the cell vacuole. Accumulation of Cl- decreases the intracellular osmotic potential. Consequently, water moves passively into the cell down a water-potential gradient. This increases cell hydration and turgor pressure, which is particularly important for maintaining leaf succulence of crops under dry conditions (e.g. sugar beet). Under saline conditions, the salt concentration outside the cell increases and the cell will plasmolyze (Figure 48, middle). This negatively affects plant growth since expansion and division of new cells seriously decreases. Plant cells adapt to this kind of water stress by osmoregulation (osmotic adjustment): they actively absorb and accumulate solutes. Increased uptake of inorganic ions decreases the cell osmotic potential, which promotes water uptake. When readily absorbable ions like K+ and Cl- are available, they are rapidly taken up and the cell water potential readjusts (Figure 48, right).
Fig. 48 Osmoregulation of a saline cell by chloride. (Left) a non-saline
cell, (middle) a saline cell and (right) a saline cell osmotically adjusted by
accumulation of Cl- (and K+ as a counter-ion) in the
vacuole. (jw:
water potential, jo:
osmotic potential, T = jw
- jo: turgor;
source: Maas, 1986a)
Chloride is not only transported in and out of cells but also across membranes within cells. This may contribute to the osmotic balance of cell organelles (e.g. chloroplasts, mitochondria) in the cytoplasm of plant cells (Maas, 1986a).
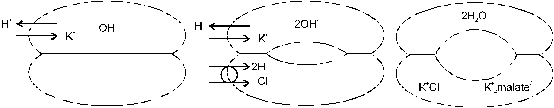
Fig. 49 Stomatal opening by ion transport in guard cells. (Left)
Excretion of protons attract K+ ions into cells, (middle) Cl- anions
are actively absorbed by a symporter, (right) absorbed Cl- and
intracellularly produced malate balance K+, and water causes swelling of the
cells (source: Maas, 1986a)
(3) Stomatal activity is an important process for CO2 assimilation and the water economy of plants. The opening-closing process of stomata depends on changes in turgor of the guard cells. Stomata open when an influx of water into the guard cells causes them to swell apart. As lined out before, increases in cell concentration of osmotic solutes decrease the cell water potential and water flows in. K+ is the dominant cation involved in stomatal movements. K+ is transferred from the vacuoles of the outer lateral subsidiary and the epidermal cells, and accumulates in the guard cells of open stomata (Mengel & Kirkby, 1987). Absorbed inorganic Cl- and the intracellularly synthesized organic acid malate are the primary anions to balance K+ in guard cells. Ion transport in guard cells of stomata functions as follows. Energy is required to extrude H+ ions from the guard cells to build up a negative charge in those cells. K+ ions are passively moved into the guard cells along the electrical potential gradient (Figure 49, left). Chloride is absorbed via the symporter previously presented in figure 11 (Figure 49, middle). Malate is synthesized in the guard cells. Accumulation of K+, Cl- and malate increases the intracellular osmotic potential and water moves into the guard cells causing them to swell (Figure 49, right). Similar mechanisms of turgor regulation may be responsible for turgor-controlled leaf movements of nyctinastic legumes (Maas, 1986a).
Presumably, all plant species use some Cl-, but many species prefer malate as counter-ions for K+. The relative amounts of Cl- and malate in guard cells depend on plant species and environmental conditions such as the external Cl- supply. Guard cells of species containing chloroplasts filled with starch can produce larger amounts of malate and thus require less chloride for optimal functioning of their leaf stomata. Among those species, corn and Commelina may use both Cl- and malate, and broad bean may prefer malate (Maas, 1986a). If the Cl- supply is sufficiently high, biosynthesis of malate in guard cells may decrease. Contrary, if supply of Cl- is low, this may trigger biosynthesis of malate. This process may be energetically more favorable since synthesis of malate requires less energy than Cl- uptake (Assmann & Zeiger, 1986).
However, plants of certain families such as Liliacea, Iridaceae, Amarylladae and Palmae possess only poorly developed chloroplasts in their guard cells and starch may be completely absent (Raschke, 1979). Those species have an absolute requirement for Cl-. von Uexküll & Sanders (1986) argued that beneficial effects of chloride on growth, yield and disease resistance of palm species may all be related to stomatal activity, osmotic potentials and water stress. Application of KCl to coconut significantly increased yield of nuts (size and weight) and copra. Palms low in Cl- suffered severe water stress resulting in frond breakage, stem cracking and stem bleeding when plants went from rapid growth in the wet season into the dry season (Table 35).
|
Table 35 Effect of KCl on Cl- leaf concentration, frond fracture and stem bleeding of coconut in North Sumatra |
|||
|
KCl level (kg/tree) |
Leaf Cl- concentration (% dry matter) |
Frond fracture (%) |
Stem bleeding (%) |
|
0 |
0.068 |
11.6 |
27.0 |
|
2.25 |
0.411 |
1.7 |
8.1 |
|
4.50 |
0.506 |
1.2 |
4.5 |
|
Source: von Uexküll & Sanders (1986) |
|||
(4) Several researchers have demonstrated the role of Cl- in photosynthesis of plants (Maas, 1986a). Cl- is required in the water-splitting reaction in Photosystem II (Hill reaction). The function of Cl- in this process may be that binding of Cl- to chloroplast membranes is necessary to activate the oxygen-evolving enzyme (Baianu et al. 1984). However, at least two reasons question significant involvement of Cl- in photosynthesis. (a) Most scientific results have been achieved in vitro. (b) The concentrations of Cl- required for optimal photosynthetic O2 evolution vary considerably among plant species: some crops like corn and spinach require 10 to 20 mM Cl- whereas halophytes require about 250 to 500 mM Cl-. Cl-concentrations may remain relatively constant in chloride-demanding plant parts regardless of whether plants are grown under deficient or excessive levels of Cl-. Even in severely stunted plants, Cl- levels in chloroplasts remain sufficiently high to maintain photosynthesis since nearly all of the Cl- in chloride-deficient plants is accumulated in the chloroplasts (Robinson & Downton, 1984). In spinach, the concentration of Cl- in the chloroplast was not significantly decreased despite a 70-percent reduction in leaf chloride. By contrast, increasing leaf-chloride concentrations 70-fold increased chloroplast-chloride less than 20 percent.
Although deficiency in Cl- may have no significant impact on photosynthesis of plants, growth of chloride-deficient plants can be markedly reduced. This may be due to effects of Cl- on enzyme activation. At least the three enzymes a-amylase, asparagine synthetase and ATPase seem to specifically require Cl- for optimal activity (Maas, 1986a). The effect of potassium chloride on ATPase may be a two-fold: the K+ stimulates the plasmalemma-bound ATPase and the Cl- stimulates the tonoplast-bound ATPase (Mengel & Kirkby, 1987). In this process, K+ largely accumulates in the cytoplasm and Cl- in the vacuole of plant cells. This may also help to explain the rapid uptake of K+ from KCl described in Chapter 3.3.
(5) Although the exact mechanisms of disease suppression of Cl- are not fully understood, application of Cl- fertilizers can reduce the severity of foliar and root diseases (Table 36). One example is the reduction in infestation of rice by stem rot and sheath blight (Table 37). Other such results have been described for cereals (Goos, 1986), and particularly wheat (Thier et al. 1986; Christensen et al. 1986; Bonczkowski, 1990).
|
Table 36 Suppression of plant diseases by chloride fertilizers |
|
|
Crop |
Pathogen |
|
winter wheat |
Take-all root rot, tanspot, stripe rust |
|
spring wheat |
leaf rust, tanspot, Septoria, common root rot |
|
barley |
common root rot, Fusarium root rot, spot blotch |
|
durum wheat |
common root rot |
|
corn |
stalk rot |
|
pearl millet |
downy mildew |
|
coconut palm |
gray leaf spot |
|
potato |
Hollow heart, brown center |
|
celery |
Fusarium yellows |
|
rice |
stem rot, sheath blight |
|
Source: Halstead et al. (1991) |
|
|
Table 37 Effect of KCl on severity of stem rot and sheath blight infestation of rice at Singamerta, Java, Indonesia |
||
|
KCl rate |
Disease severity |
|
|
(kg K2O/ha) |
stem rot (%) |
sheath blight (%) |
|
0 |
36.9 |
25.6 |
|
16 |
30.6 |
21.2 |
|
36 |
25.8 |
16.0 |
|
54 |
23.1 |
12.8 |
|
Source: Ismunadji et al. (1990) |
||
Many of those effects may be associated with improved stomatal regulation and associated reductions in water stress, improvements in plant nutritional status and improved rhizosphere soil conditions through inhibition of nitrification (Christensen et al. 1990; Fixen, 1987; Wang, 1987).
4.6 Chloride tolerance
Chloride tolerance of plants differs considerably among plant species and even different cultivars of the same species. Tolerance stands for different levels of plant response to chloride ranging from requirement to toxicity.
Chlorophilic plant species, which require chloride for maintaining stomatal activity, are among the Liliacea, Iridaceae, Amarylladae and Palmae as outlined before. Halophytes accumulate ions for increasing the osmotic potential and decreasing the water potential of their tissues to promote water uptake under saline conditions. Some species regulate their tissue salt concentration by excreting excess salts through salt glands or bladder hairs, increasing their succulence, and sequestering ions in the vacuoles.
Plant genera or genotypes, which do not require but tolerate higher levels of chloride, primarily differ from intolerant species in their ability to (1) restrict chloride absorption, (2) restrict Cl- translocation from roots to shoots, and (3) distribute chloride within shoots. (1) Some chloride tolerant species/cultivars restrict Cl- entry into the root e.g. by presence of efficient barriers. Alternatively, absorbed Cl- is effluxed back into the growth medium. (2) Others species retain more Cl- in the roots and contain lower Cl- in shoots. This may be due to restricted ion release into the xylem but also to re-translocation of Cl- from shoots to roots. (3) Selective distribution of Cl- in plants leading to accumulation of Cl- in specific organs such as vacuoles, older plant parts and shed organs rather than cell cytoplasm or meristems is another tolerance mechanism in plants (Aswathappa, 1987). Table 38 summarizes a number of mechanisms of ion regulation in chloride tolerant species.
|
Table 38 Possible mechanisms of Cl- regulation by chloride tolerant plant species |
|
· restriction of Cl- entry into the root |
|
· at the root exodermis |
|
· at the root endodermis |
|
· efflux of Cl- from roots into the growth medium |
|
· reduced xylem loading |
|
· re-absorption and retention |
|
· in the proximity of roots |
|
· at the basal stem |
|
· localization in older leaves |
|
· compartmentation in cells |
|
· succulence |
|
· leaf extrusion |
|
· retention in the petiole |
|
Source: Aswathappa (1987) |
A large number of crops are susceptible to excessive chloride. Most detrimental effects of Cl- result from its contribution to overall osmotic stress in plants caused by excessive salts in the root medium (Chapter 3.6). However, several economically important crops are chloride intolerant for reasons other than osmotic stress. Symptoms in those chlorophobic crops reach from reductions in yield and quality to injuries caused by chloride toxicity.
Chloride tolerance in soybean depends on the ability of cultivars to prevent or retard Cl- accumulation in tops (Maas, 1986a). This tolerance may vary substantially: chloride susceptible cultivars accumulate considerable amounts of Cl- in leaves and seed (Parker et al. 1986). Consequently, they develop toxicity symptoms expressed as leaf scorch, yields remain low or the crop fails completely (Table 39). Salt tolerant cultivars may be able to reabsorb Cl- from the xylem in the basal part of the roots. Similar observations have been made for beans (Phaseolus spp.; Mengel, 1991) and may also be true for peanut (Aljibury & Talabany, 1982).
|
Table 39 Effect of chloride on susceptible and tolerant soybean cultivars |
||||
|
Cultivar |
Plant Cl- concentration |
Leaf scorch |
Yield |
|
|
|
leaves (%) |
seed (ppm) |
(rating) a |
(bu/A) |
|
chloride susceptible |
1.67 |
682 |
3.4 |
15 |
|
chloride tolerant |
0.09 |
111 |
1.0 |
24 |
|
a 1 = none, 2 = mild, 3 = moderate, 4 = heavy, 5 = severe damage Source: Parker et al. (1986) |
||||
In sugarcane, the Cl- ion adversely affects sugar content due to its electrical charge and role as a counter-ion (Table 40).
|
Table 40 Effect of chloride on sucrose content in sugarcane |
||
|
Stem portion |
Cl |
Sucrose |
|
|
(% in dry matter) |
|
|
upper part |
1.40 |
|
|
central part |
0.84 |
16.1 |
|
basal part |
0.70 |
31.8 |
|
Source: Krauss (1991) |
||
Generally, sucrose synthesized in the leaves is translocated via the phloem stream and unloaded into the storage cells located in the stem. Excessively absorbed chloride is translocated to leaves and due to the low metabolic requirement of leaves for chloride, re-translocated via the phloem and sequestered into the vacuoles of storage cells in the stem. For electrical charge neutrality, the Cl- anion is accompanied by the K+ cation which also accumulates in the storage tissue. This has a two-fold effect on storage of sucrose in sugarcane: (1) K+ and Cl- raise the osmotic potential of storage cells in sugarcane stems and impede sucrose transport into the storage cells. Moreover, the ions compete with sucrose for storage space in the cell vacuoles. (2) Cl- decreases the pool of plant potassium available for other metabolic functions in the plant (Krauss, 1991).
The same metabolic relations may be true for the adverse effect of Cl- on quality of pineapple. KCl decreased dry extract (a measure of sugar content) and flesh color, increased acidity, and reduced quantitative yield as compared to K2SO4 (Table 41). At the same time, Cl- may cause leaf scorch in certain conditions (Marchal et al. 1981).
|
Table 41 Effect of KCl and K2SO4 on yield and quality parameters of pineapple |
||
|
Parameter |
KCl |
K2SO4 |
|
acidity (me/l) |
||
|
dry extract (°Brix) |
14.8 |
15.0 |
|
fruit weight (kg) |
1.69 |
1.79 |
|
Source: Lacoeuilhe (1979) |
||
Due to its high K demand, many studies have been conducted on the effects of different potash fertilizers on potato (see also Chapter 4.4.5). As compared to K2SO4, application of KCl usually results in greater shoot growth (low tuber:shoot ratio) but delayed tuber development, lower starch percentage in tubers and lower yields (Orlovius, 1996; Table 42). At the same time, the water content of shoots and tubers is higher with potassium chloride.
|
Table 42 Effect of KCl and K2SO4 on yield attributes of potato |
|||
|
Attribute |
Control |
KCl |
K2SO4 |
|
tuber:shoot ratio |
1.2 |
2.2 |
3.6 |
|
starch content (% fresh weight) |
12.5 |
12.5 |
14.0 |
|
tuber weight (g dry weight/pot) |
78 |
125 |
163 |
|
Source: Beringer & Mutert (1991) |
|||
Stimulation of shoot growth with KCl is very likely a consequence of the preferential compartmentation of absorbed Cl- in the shoots and the effects of the chloride ion as an osmotic solute on the water economy of potato (Beringer et al. 1990). Table 43 shows the effects of chloride on attributes of cell sap in potato leaves.
|
Table 43 Effects of KCl and K2SO4 on attributes of cell sap in potato leaves |
||
|
Attribute |
KCl |
K2SO4 |
|
osmotic potential jo (MPa) |
-1.24 |
-0.95 |
|
water content (g H2O/g dry weight) |
7.8 |
6.2 |
|
Source: Beringer & Mutert (1991) |
||
As indicated by the lower osmotic potential, Cl- increased the turgor potential, which increased water uptake of leaf cells. The greater turgidity of cells suggests greater cell extension and consequently higher sink strength of the shoot compared with the tuber.
Another process may be responsible for adverse effects of chloride on starch content and specific gravity in potato. Due to the low physiological requirements of potato for chloride and the effect of the Cl- anion on balancing charges in plant cells, nutrient ions may be tied-up and thereby made unavailable for metabolic processes. With greater Cl concentrations, concentrations of N and K in tuber ends were greater, but starch concentration and specific gravity lower (Westermann et al. 1994a; Westermann et al. 1994b). Therefore, petiole NO3 and K concentrations were negatively related to specific gravity.
Freedom from chloride makes potassium sulfate superior to potassium chloride for tobacco, where the intrinsic value is as important as total yield (von Peter, 1981). Leaf chloride drastically reduces tobacco quality (Tandon & Kemmler, 1986; Shah, 1987). Chloride decreased yields and had a negative effect on most quality parameters of tobacco in several tobacco growing districts in Spain (Table 44; Llanos Company, 1984).
|
Table 44 Effects of KCl and K2SO4 on yield and quality parameters of tobacco |
||
|
Parameter |
KCl |
K2SO4 |
|
yield (kg/m2) |
0.163 |
0.195 |
|
combustibility |
7.43 |
13.28 |
|
filling power |
36.35 |
34.82 |
|
chlorine |
2.37 |
0.94 |
|
ash |
25.34 |
24.89 |
|
total N |
3.47 |
4.08 |
|
total volatile bases (TVB) |
0.676 |
0.690 |
|
nicotine |
1.00 |
1.17 |
|
sulfur |
0.29 |
0.32 |
|
protein N |
1.82 |
1.54 |
|
potassium |
5.58 |
5.98 |
|
calcium |
7.71 |
7.18 |
|
magnesium |
1.37 |
1.22 |
|
pH |
6.25 |
6.23 |
|
Source: Llanos Company (1984) |
||
KCl distinctively increased the chlorine content of the leaf which was correlated with the shorter persistence of burning. This negative effect on combustibility of tobacco is related to the hygroscopic effect of Cl- making drying and fermentation difficult.
Besides satisfying its high S requirements, the sulfate from K2SO4 improves amino acid content and nitrate reduction in tea compared to potassium chloride (Chapter 4.4.2). By contrast, the chloride from KCl has an inhibiting effect on nitrate reduction and synthesis of amino acids (Table 45) and may cause leaf scorch (Rahman et al. 1978).
|
Table 45 Effects of KCl on nitrate reduction and amino acids in tea seedlings |
||
|
Fertilization |
Nitrate reduction (%) |
Amino acids (%) |
|
without KCl |
100 |
100 |
|
with KCl |
79 |
91 |
|
Source: Wu (1995) |
||
Unlike herbaceous crops, woody plant species are generally susceptible to chloride. Their tolerance can be improved by selecting rootstocks that restrict accumulation of chloride in the shoots by limiting absorption and translocation of chloride. However, avoiding excessive chloride inputs through appropriate fertilization strategies may be the better alternative.
Prasad et al. (1993) mentioned the detrimental effects of Cl- on kiwifruit. Marginal leaf scorch and leaf necrosis developed and increased with higher leaf Cl- concentrations at higher KCl rates. Breakdown symptoms could not be eliminated by splitting KCl applications and were even more severe with greater rates of N application. Such effects are probably due to the inability of plants to decrease absorption and translocation of Cl- into the shoots, or to sequester leaf chloride into vacuoles.
Table 46 shows the detrimental effect of Cl- on grape (vine). Yields decreased with increasing proportion of Cl- in a KCl/K2SO4 nutrient solution.
|
Table 46 Effect of KCl and K2SO4 on yield of grape in nutrient solution |
|
|
Proportion KCl:K2SO4 |
Weight per bunch (g) |
|
4.0:0.0 |
58.9 |
|
2.5:1.5 |
70.5 |
|
1.0:3.0 |
84.2 |
|
0.0:4.0 |
91.3 |
|
Source: Edelbauer (1979) |
|
In India, the continuous use of chloride-containing fertilizers resulted in widespread chloride toxicity in grape (Tandon & Kemmler, 1986). Grape was found unable to adjust to chloride salinity and showed toxicity symptoms such as chlorosis and necrosis of leaves, early leaf abscission, die-back and decline (Satyanarayana, 1981). Such grapes have only poor post-harvest quality (Chadha, 1984).
Incidence of premature fruit drop, blight, chlorosis and leaf damage in citrus have been associated with chloride toxicity (Zehler et al. 1981). Ben-Hayyim & Kochba (1983) showed that toxic effects in callus cells of citrus are due to accumulation of Cl-. Resistant cell lines had bigger vacuoles to sequester chloride under salt stress. Bar et al. (1996) attributed leaf damage and reduced branch growth in two citrus rootstocks to increased putrescine and decreased spermine contents caused by accumulation of chloride in citrus leaves. Greater putrescine content may be related to ethylene production in citrus.
Similar observations have been made for coffee. Cl- in leaves rapidly increased with increasing rates of potassium chloride (Gonzales et al. 1977). High chloride levels in the leaves were related to reduced growth, necrosis, and defoliation (Snoeck et al. 1986; Furlani, 1978).
5.1 Sulfur and chloride in soils
Availability of nutrients to plants depends on their concentration in the soil liquid phase. Sulfur is available to plants as inorganic SO42- in the soil liquid phase. Most of this S is supplied by organic S fractions in soil with only a small part of the sulfur residing in the soil biomass. Many of the microbial transformations of organic and inorganic S-forms in soil are not fully understood, but maintenance of soil organic matter and adequate S fertilization are both means to supply S to crops.
Under humid or tropical conditions, contents of sulfate in agricultural soils are oftentimes low. This is due to low organic matter, high leaching, and adsorption of SO42- in acid soils. Under such conditions, plant-available SO42- must be supplied since additions of other S forms (e.g. elemental S) may further increase soil acidity. The latter process is, however, desirable to decrease the high pH and improve physical and biological properties of alkaline soils.
Under flooded soil conditions, availability of S to plants can be negatively affected by microbial immobilization of SO42- into organic S. Reduction of sulfate to sulfide in reduced soil layers may be potentially harmful to crops, but many authors have recommended application of K2SO4 to crops cultivated in flooded soils (i.e. rice).
As an ion with low adsorption capacity, soil chloride is more readily leached than sulfate. However in coastal regions, arid and semi-arid zones, and when high doses of Cl-containing fertilizers are applied, Cl- can accumulate. Application of KCl to soils in such and other regions invariably increases soil salinity due to the high salt index of KCl.
5.2 Absorption of sulfur and chloride by plants
Absorption of nutrients by plants depends on the nutrient concentration and other soil conditions in the near vicinity of their roots (i.e. rhizosphere). Roots of certain crops (e.g. barley) exude organic compounds which increase oxidation of S to plant-available SO42-. Due to the acidifying effect of oxidation of elemental sulfur to sulfate, root growth of crops in alkaline soils can be improved.
In contrast to the passive absorption of cations such as K+, the anions SO42- and Cl- are absorbed actively. Therefore, their concentration in plant tissues can exceed concentrations in soil many times. Two mechanisms have been supposed for this: (1) antiport mechanism and (2) symport mechanism.
The monovalent Cl- anion is absorbed more rapidly than the bivalent SO42- anion. Therefore the K+ cation from KCl is faster assimilated than the K+ from K2SO4. Absorption of K+ and Cl- from KCl occurs at similar rates, but K+ from potassium sulfate is absorbed at a faster rate than SO42-. Therefore, plants fertilized with K2SO4 have to excrete H+ protons to balance slower absorbed SO42- anions in the soil solution. This temporarily decreases the pH in the rhizosphere and has been regarded beneficial to improve availability of other essential nutrients (e.g. P). The resulting excess of K+ cations in plants is balanced by organic acids (e.g. malate). This triggers photosynthesis since CO2 is required for synthesis of such organic acids.
Rapid assimilation of Cl- has a competitive effect on absorption of other anions and particularly NO3 and SO4. This may have a desired effect on reducing concentration of certain ions in plants (e.g. nitrate in leafy vegetables), but may hinder optimal nutrition of crops. On the other hand, Cl- may increase absorption of counter-ions such as calcium.
Absorption of S and N is interrelated: additions of N improve S uptake of crops and vice versa. Therefore, K2SO4 is a good supplement of nitrogenous fertilizers. Such synergetic effects are also relevant for other nutrients (K, P, etc.).
Many crops such as vegetables are susceptible to high ion concentration of the soil solution. They may be more susceptible at germination or after germination. For such crops, K2SO4 is the preferred K-fertilizer, since the salt index of potassium sulfate is much lower than the salt index of potassium chloride.
5.3 Sulfur and chloride in plants
Compared with chloride which is highly mobile in plants and can be translocated in akropetal and basipetal direction, sulfate is mainly transported in akropetal direction, initially to older plant parts. If their demand is satisfied, SO42- is translocated to younger leaves and meristems. Therefore, S deficiency symptoms appear first on the younger leaves and not on the older leaves as for nitrogen. At low S supply, inorganic S is rapidly incorporated into organic S compounds and only accumulates in tissues at high supply. Presence of inorganic S in plants, therefore, indicates sufficient S supply.
Assimilation of SO42- requires enzymes located in the membrane of chloroplasts. Hence assimilation of sulfate depends on photosynthesis. Sulfatetransferase and ATPreductase are important enzymes and Ferredoxin an important electron donator for activation and reduction of SO42-.
Cysteine is the first stable organic S form and precursor of other amino acids and S-containing biochemical compounds. The latter may be divided into chemical categories according to type of S bond: (1) sulfide bonds occur in S-containing amino acids which account for the bulk of the S in plants. Cysteine, methionine and acetyl-CoA are important substances containing sulfide bonds. Sulfide bonds can be found in volatile S compounds such as leek oils (e.g. in Alliaceae) and mustard oils (e.g. in Cruciferae) which are of great significance in horticultural production. (2) disulfide bonds are essential for polymerization of polypeptides and proteins and play a central role in the cysteine, glutathione and lipoic acid redox systems of plants. The latter are essential for plant metabolism. (3) Rhodanide-type bonds occur in plants as mustard-oil glucosides or glucosinolates. Upon hydrolysis, isothiocyanates (e.g. mustard oil) are formed. (4) Thiamin (vitamin B1) and biotin (vitamin H) are important heterocyclic S compounds, which occur largely in grains of cereals and legumes. For agricultural purposes, these S-containing organic compounds should preferably be separated into substance categories (Table 22).
Sulfur is ultimately connected with photosynthesis of plants since assimilation of absorbed S is carried out by enzymes in the membrane of chloroplasts and the S-containing organic compounds ferredoxin and acetyl-CoA play a significant role in photosynthesis. S deficiency affects chloroplast formation and may even cause their decomposition.
Except plant species which contain large amounts of leek and mustard oils, the bulk of organic S in plants consists of amino-acid and protein S. S deficiency inhibits polymerization of proteins, and non-S-containing soluble amino acids and nitrate accumulate in plants. The growth of protein-rich crops like legumes is consequently inhibited. For these crops, S-fertilization can improve yields and protein contents substantially.
In plant species capable of synthesizing lipids (oil crops), they are the primary storage form of S. S-fertilization can improve yield and oil content, and quality of oil in these crops. Due to its involvement in carbohydrate metabolism, sulfur positively affects sugar contents of crops (e.g. sugarcane). K and S improve synthesis of starch from sugars. Therefore, application of K2SO4 may decrease sugars, but increase starch in crops. This can improves yield and quality of starch crops, e.g. potatoes. Yields and carbohydrate contents of rice may be significantly improved by potassium sulfate.
Instead of inhibiting uptake of NO3 by crops (Cl-, see above), S can lower nitrate contents in crops by improving incorporation of N into organic compounds: (1) S promotes chloroplast formation and photosynthesis, (2) the S-rich protein ferredoxin is involved in nitrate reduction, and (3) sufficient S prevents accumulation of non-S amino acids which depress the activity of nitrate reductase.
Chloride functions in plants as (1) a counter-ion for cation transport, (2) an osmotic solute, (3) a regulator for stomatal activity, (4) an ion participating in metabolic processes in plants, and (5) an ion active in suppressing disease infections. (1) As a counter-ion, Cl- may increase absorption of cations (e.g. K+), but may tie up cations required for metabolism in plants. (2) By decreasing their intracellular osmotic potential, chloride acts as an osmotic solute to promote water uptake of plants. This is advantageous for halophytes or crops grown under dry conditions. However for tuber crops such as potato, increased water uptake of crops may stimulate shoot growth for the expense of tuber formation. (3) K+, Cl- and malate are osmotic solutes for regulation of stomatal activity. Cl- and malate act as counter-ions for K+ which accumulates in guard cells of stomata, raises their osmotic potential and causes them to swell. Most plant species may prefer malate as a counter-ion for K+ but some species (e.g. Liliaceae, Iridaceae, Amarylladae and Palmae) have an absolute requirement for chloride due to their poor ability to synthesize malate in the guard cells of their stomata. (4) Although only very low quantities may be required, chloride is essential for photosynthesis of plants (Hill reaction). Cl- is involved in other metabolic processes in plants such as enzyme activation. (5) Although the exact mechanisms of disease suppression of chloride are not known, application of Cl- fertilizers can reduce the severity of certain diseases.
Chloride tolerance varies among plant species. Chlorophilic plant species like halophytes require chloride for increasing their osmotic potential or maintaining stomatal activity (see above). Cl- tolerant species may be able to (1) restrict absorption of chloride, (2) restrict translocation of chloride from roots to shoots, or (3) selectively distribute Cl- in specific organs. However, many important crops are chloride intolerant or their quality is negatively affected by Cl-. Among those are soybean, sugarcane, pineapple, potato, tobacco, tea, kiwifruit, grape, citrus, and coffee.
Abd-Elfattah, A, Hilal, MH, El-Habbasha, KM, Bakry, MD, 1990: Amendment of alkaline clay soil by elemental sulphur and its effect on the response of garlic to phosphorus and nitrogen. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 295-313.
Abdel-Samad, Ismail, S, El-Mashhadi, HHM, Radwan, SA, 1990: Effect of the interactions between leaching processes with sulphur and peat on growth and uptake of nutrients by barley grown in saline calcareous soils. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 325-337.
Abu-Zeid, MI, 1992: The utilization of N-urea by wheat grown on sandy calcareous soil as affected by adding potassium. Assiut J. Agri. Sci. 23, 265-279.
Adetuni, MT, 1992: Effect of lime and phosphorus application on sulphate-adsorption capacity of south-western Nigerian soils. Indian J. Agric. Sci. 62, 150-152.
Aljibury, LK, Talabany, D, 1982: Effect of different salinity levels on yield and oil content of peanut seeds. J. Res. Agric. Water Resources 1, 121-127.
Amarasiri, SL, 1987: A status report on sulphur in Sri Lankan agriculture. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 83-88.
Amberger, A, 1979: Pflanzenernährung. Verlag Eugen Ulmer, Stuttgart.
Anonymous, 1979: Pineapples thrive on potassium. Information Bulletin, Citrus and Subtropical Fruit Research Institute (South Africa) 78, 10-11.
Assmann, SM, Zeiger, E, 1986: Guard cell bioenergetics. In: E Zeiger, G Farquhar, I Cowin (eds): Stomatal Function. Stanford University Press, Stanford.
Aswathappa, N, 1987: Salt Tolerance of Casuarinas with Special Reference to Ion Regulation. Australian National University, Canberra.
Baianu, IC, Critchley, C, Govindjee, Gutowsky, HS, 1984: NMR study of chloride ion interactions with tylakoid membranes. Proc. Nat. Acad. Sci. 81, 3713-3717.
Bakhsh, A, Khattak, JK, Bhatti, AU, 1986: Comparative effect of potassium chloride and potassium sulfate on the yield and protein content of wheat in three different rotations. Plant and Soil 96, 273-277.
Bar, Y, Apelbaum, A, Kafkafi, U, Goren, R, 1996: Polyamines in chloride-stressed Citrus plants: alleviation of stress by nitrate supplementation via irrigation water. J. Am. Soc. Hort. Sci. 121, 507-513.
Barker, AV, Mills, HA, 1980: Ammonium and nitrate nutrition of horticultural crops. Hort. Rev. 2, 395-423.
Beaton, JD, Pretty, KM, Sanders, JL, 1988: The chloride component of fertilizers can be beneficial. Third Chemical Congress of North America, Toronto.
Ben-Hayyim, G, Kochba, J, 1983: Aspects of salt tolerance in a NaCl-selected stable cell line of Citrus sinensis. Plant Phys. 72, 685-690.
Beringer, H, Koch, K, Lindhauer, MG, 1990: Source:sink relationships in potato (Solanum tuberosum) as influenced by potassium chloride or potassium sulphate nutrition. In: ML van Beusichem (ed.): Plant Nutrition Physiology and Application. Kluwer Academic Publishers, 639-642.
Beringer, H, Mutert, E, 1991: Sulphur nutrition of crops with special reference to K2SO4. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 62-69.
Bhuijan, NI, 1991: Sulphur fertilization for rice and non-rice crops of Bangladesh agriculture. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 81-93.
Biswas, BC, Tewatia, RK, 1991: Role of sulphur in balanced plant nutrition Indian experience. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 98-106.
Blair, GJ, 1986: Sulfur cycling in flooded rice. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 453-468.
Blair, GJ, Lefroy, RDB, 1987: Sulphur cycling in tropical soils and the agronomic impact of increasing use of S free fertilizers, increased crop production and burning of crop residues. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 12-17.
Blair, GJ, Lefroy, RDB, Chaitep, W, Santoso, D, Samosir, S, Dana, M, Chinoim, N, 1991: Matching sulfur fertilizer to plant production systems. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 156-175.
Bohn, HL, Barrow, NJ, Rajan, SSS, Parfitt, RL, 1986: Reactions of inorganic sulfur in soils. In: MA Tabatabai (ed.): Sulfur in Agriculture. Agronomy Series No. 27. ASA, CSSA, SSSA, Madison, 233-249.
Bonczkowski, LC, 1990: Response of hard red winter wheat to chloride application in eastern Kansas. Kansas State University, Manhattan.
Brunet, R, Treto, E, 1988: Some aspects of potassium and magnesium in a compact red ferrallitic soil. Occasional Publication, Instituto Superior de Ciencias Agropecuarias de la Habana No. 1512.
Callan, NW, Westcott, MP, 1996: Drip irrigation for application of potassium to tart cherry. J. Plant Nutr. 19, 163-172.
Chadha, KL, 1984: Grape research in Indiapriorities and suggested approach for future. Indian J. Hort., 145-159.
Chaiwanakupt, S, Parkpian, P, Pongsakul, P, Phetchawee, S, 1987: Sulphur status and requirements in Thailand. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 89-94.
Chowdhury, IR, Paul, KB, Eivazi, F, Bleich, D, 1985: Effects of foliar fertilization on yield, protein, oil and elemental composition of two soybean varieties. Com. Soil Sci. Plant Anal. 16, 689-692.
Chowdhury, MAH, Majumder, AK, 1994: Effect of some sources of sulphur on the yield and yield attributes of rice. Bangladesh J. Scientific Ind. Res. 29, 185-191.
Christensen, NM, Brett, MA, Hart, JM, Weller, DM, 1990: Disease dynamics and yield of wheat as affected by take-all, N sources, and flourescent Pseudomonas. In: Trans. 14th Int. Congress of Soil Sci. Vol. 111, Kyoto, 10-15.
Christensen, NM, Roseberg, RJ, Brett, M, Jackson, TL, 1986: Chloride inhibition of nitrification as related to take-all disease of wheat. In: Chloride and Crop Production. Potash and Phosphate Institute. Spec. Bull. No. 2, Atlanta, 22-39.
Collins, M, Duke, SH, 1981: Influence of potassium-fertilization rate and form on photosynthesis and N2 fixation of alfalfa. Crop Sci. 21, 481-485.
Csinos, AS, Gaines, TP, 1986: Peanut pod rot complex: a geocarposphere nutrient imbalance. Plant Disease 70, 525-529.
Davide, JG, Nabhan, H, Saleem, M, Ahmad, N, 1986: Potash fertilizers in Pakistan: sulphate and muriate of potash. Publication, Nat. Fert. Dev. Centre, Pakistan, No. 7.
Edelbauer, A, 1979: Investigations on the influence of varied KCl/K2SO4 ratios on yield of grapes, and on quality of grape juice and its amino acid pattern, of Vitis vitifera in nutrient solution culture. Potash review. Subject 29, Suite 10.
Elam, M, Ben-Ari, S, Magen, H, 1995: Dissolving different potassium fertilizers for fertigation. Int. Water & Irrigat. Rev. 15, 27-29.
El-Bahr, MK, 1993: Responses of chamomile callus cultures to potassium and sodium sulphate salinity. Egyp. J. Hort. 20, 15-22.
El-Desoky, MA, Dawood, RA, El-Far, IA, 1993: Response of lentil grown on broad ridges to irrigation schedules and potassium fertilization. Assiut J. Agric. Sci. 24, 51-72.
El-Fouly, MM, Fawzi, AFA, El-Baz, FK, 1989: Concentration and uptake of N, P and K and the effect of potassium sulphate on Vicia faba L. FABIS Newsletter 25, 18-22.
El-Gengaihi, S, Turkey, KA, Shalaby, AS, Ibrahim, NA, 1995: effect of fertilization treatments on yield parameters and mucilage and fat contents in roots of moghat (Glossostemon bruguiri Desf.). Plant Foods Human Nutr. 47, 239-244.
El-Leboudi, A, Abu-Hussin, H, Youssef, G, 1993: Dynamics of K uptake by potato slices under conditions of nitrogen fertilization. Annals Agric. Sci. 38, 345-355.
Fahmy, L, Hassaballa, IA, 1977: The effect of different rootstocks and potassium sulfate fertilizer on leaf potassium, calcium and sodium in young Clementine. Lybian J. Agric. 6, 1-5.
Farrag, AA, Shehata, AA, Kandil, MM, 1990: The effect of phosphorus and sulphur fertilizers on seed protein of broad bean plants. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 361-371.
Fixen, PE, 1987: Chloride fertilization: recent research gives new answers. Crops and Soils 39(6), 14-16.
Fox, RL, Hue, NV, 1986: Sulfur cycling in the tropics and sulfur requirements for agriculture. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 139-164.
Freney, JR, 1986: Forms and reactions of organic sulfur compounds in soil. In: MA Tabatabai (ed.): Sulfur in Agriculture. Agronomy Series No. 27. ASA, CSSA, SSSA, Madison, 207-232.
Fried, M, Shapiro, RE, 1961: Soil-plant relationships in ion uptake. Ann. Rev. Plant Physiol. 12, 91-112.
Furlani, AMC, 1978: Effects of potassium chloride and of potassium sulfate on coffee plants. Fertilizer Abstracts II(4), 125.
Furlani, AMC, Catani, RA, de Moraes, FRP, Franco, CM, 1976: The effects of potassium chloride and potassium sulphate applications on coffee nutrition. Bragantia 35, 349-364.
Germida, JJ, 1990: Microbial oxidation of elemental sulphur fertilizers in agroecosystems: a review of studies on western Canadian prairie soils. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 125-146.
Gonzales, MA, Lopez, CA, Carvajal, JF, Bricenõ, JA, 1977: Effect of potassium sources on the accumulation of chlorides and sulfates in coffee trees. Soils and Fertilizers 40, 9.
Goos, RJ, 1986: Effects of KCl fertilization on small grains in North Dakota. In: TL Jackson (ed.): Chloride and Crop Production. Potash and Phosphate Institute, Atlanta, 52-61.
Goos, RJ, Holmes, BM, Johnson, BE, 1987a: Effect of chloride fertilization on cereal diseases in North Dakota. In: 30th Annual Manitoba Soil Sci. Meet., Winnipeg, 186-190.
Goos, RJ, Holmes, BM, Johnson, BE, 1987b: Effect of potassium chloride fertilization on two barley cultivars differing in common root rot reaction. Can. J. Plant Sci. 67, 395-401.
Goraya, DS, Singh, R, Brar, SPS, 1984: Response of linseed and cowpeas to different sources of sulphur in neutral and acid soils of Himachal Pradesh. Himachal J. Agric. Res. 10, 67-69.
Gupta, VVSR, Lawrence, JR, Germida, JJ, 1988: Impact of elemental sulfur fertilization on agricultural soils. I. Effects on microbial biomass and enzyme activities in soils. Can. J. Soil Sci. 68, 463-473.
Gurmani, AH, Bhatti, A, Rehman, H, 1984: Potassium fertilizer experiments in farmer fields. International Rice Research Newsletter IRRN 9, 3, 26.
Haeder, HE, 1976: Einfluß chloridischer und sulfatischer Ernährung auf Assimilation und Assimilatverteilung in Kartoffelpflanzen. Landw. Forsch. Sonderheft 32/I., 122-131.
Hähndel, R, Isermann, K, 1993: Soluble nitrogen and carbon in the subsoil in relation to vegetable production intensity. Acta Hort. 339, 193-206.
Halstead, EH, Beaton, JD, Keng, JCW, Wang, M, 1991: Chloride: new understanding of this important plant nutrient. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 314-325.
Hanson, AD, Wyse, R, 1982: Biosynthesis, translocation of betaine in sugar beet and its progenitors in relation to salinity. Plant Physiol. 70, 1191-1198.
He, T, He, C, Härdter, R, 1994: The effects of K and Mg fertilizers applied to cabbage on yield, quality and economic returns. Potash review, Subject 9, 7th Suite, No. 2.
Hiatt, AJ, 1967: Reactions in vitro of enzymes involved in CO2 fixation accompanying salt uptake by barley roots. Z. Pflanzenphysiol. 56, 233-245.
Hilal, MH, 1990: Sulphur in desert agro-sytems. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 19-50.
Hilal, MH, Abdel-fattah, A, KorKor, SA, 1990a: Effect of fine and granular sulphur application on root depth and yield of Lupinus in sandy soils. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 207-216.
Hilal, MH, El-Lakkany, H, El-Sheemy, H, 1990b: Effect of sulphur and long term fertilizer application program on rhizosphere activity and yield of peanuts in a sandy soil. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 217-227.
Hilal, MH, Selim, AM, El-Neklawy, AS, 1990c: Enhancing and retarding effects of combined sulphur and P fertilizers applications on crop production in different soils. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 281-289.
Hilal, MH, Selim, AM, KorKor, SA, 1990d: Response of peas to application of sulphur-urea mixtures in sandy and clay loam soils. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 351-359.
Hocking, PJ, Pinkerton, A, Good, A, 1996: Recovery of field-grown canola from sulfur deficiency. Austr. J. Exp. Agric. 36, 79-85.
Horsnell, LJ, 1985: The growth of improved pastures on acid soils. 3. Response of lucerne to phosphate as affected by calcium and potassium sulfates and soil aluminium levels. Austr. J. Exp. Agric. 25, 557-561.
Huang, HC, Tsai, YF, Lay, WL, 1989: Studies on the soil retarded growth factors of vegetable farms in Central Taiwan. Bul. Taichung Dist. Agric. Impr. St. 24, 63-70.
Ikeda, H, 1991: Utilization of nitrogen by vegetable crops. Jap. Agric. Res. Quart. 25, 117-124.
Islam, MS, 1986: Sulphur in Bangladesh Agriculture. In: S Portch (ed.): Proceedings: Sulphur in Agricultural Soils. Bangladesh Agricultural Research Council, Dhaka.
Ismunadji, M, 1991a: Sulfur nutrition of food crop based farming systems in Indonesia. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 70-77.
Ismunadji, M, 1991b: S research on rice in Indonesia. In: G Blair, R Lefroy (eds): Sulfur fertilizer policy for lowland and upland rice cropping systems in Indonesia. Austr. Center for Int. Agric. Res., Canberra, 87-90.
Ismunadji, M, Makarim, AK, Sudaryono, 1990: Potassium as a component of balanced fertilization in food crop production. Presented at the Symposium on Maximum Yield Research. Kyoto, 17 Aug.
Jackson, TL, McBride, RE, 1986: Yield and quality of potatoes improved with potassium and chloride fertilization. In: TL Jackson (ed.): Chloride and Crop Production. Potash and Phosphate Institute, Atlanta, 73-83.
Karwasra, SPS, Raj, M, 1984: Yield and sulphur uptake of green gram as affected by sources and levels of sulphur. Int. J. Trop. Agric. 2, 331-335.
Kennedy, IR, 1986: Acid Soil and Acid Rain. Research Studies Press Ltd., John Wiley & Sons Inc., New York.
Khamis, MA, Gomaa, AH, Sharaf, MM, Kandil, EA, 1994: Comparative studies on mineral fertilization of some deciduous fruit rootstocks: III. Potassium fertilization. Bul. Fac. Agric. Univ. of Cairo 45, 877-894.
Kirkby, EA, 1968: Influence of ammonium and nitrate nutrition on the cation-anion balance and nitrogen and carbohydrate metabolism of white mustard plants grown in dilute nutrient solutions, Soil Sci. 105, 133-141.
Kleinhenz, V, 1997: Technologies for Sustainable Vegetable Production in the Tropical Lowlands. Herbert Utz Verlag, München.
Kleinhenz, V, Schnitzler, WH, Midmore, DJ, 1997: Seasonal effects of soil moisture on soil N availability, crop N status, and yield of vegetables in a tropical, rice-based lowland. Der Tropenlandwirt 98(April 97), 25-42.
Krauss, A, 1991: Chloride and sucrose content in sugarcane. 1991 IFA-FADINAP Regional Fertilizer Conference for Asia and the Pacific, 8-11 December 1991, New Delhi.
Krishnamurti, GSR, Huang, PM, 1987: The influence of potassium chloride on the kinetics of manganese release from soils representing different taxonomy orders. In: Agronomy Abstracts. 1987 Annual Meets., Atlanta, 29 Nov.-4 Dec. ASA, Madison, 170.
Lacoeuilhe, JJ, 1979: N-K manuring of the pineapple in the Ivory Coast. Potash review, Subject 27, Suite 89.
Lin, W, 1981: Inhibition of anion transport in corn root protoplasts. Plant Physiol. 68, 435-438.
Liu, C, Cao, S, Wu, X, 1993: Sulphur in the Agriculture of China. Sulphur in Agriculture 17, 3-7.
Liu, Z, 1986: Preliminary study of soil sulfur and sulfur fertilizer efficiency in China. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 371-388.
Llanos Company, M, 1984: Düngungsversuche mit Tabak. Kali Briefe 5, Fachgebiet 12, 23. Folge.
Luisi, MVV, Rossiello, ROP, Fernandes, MS, 1983: Forms of nitrogen and levels of potassium on the absorption of phosphorus in maize. Pesquisa Agropecuaria Brasileira 18, 343-350.
Maas, EV, 1986a: Physiological response of plants to chloride. In: TL Jackson (ed.): Chloride and Crop Production. Potash and Phosphate Institute, Atlanta, 4-20.
Maas, EV, 1986b: Salt tolerance of plants. App. Agric. Res. 1, 12-26.
Malik, RS, Karwasra, SPS, Khera, AP, 1992: Effect of chloride and bicarbonate on sulphur uptake and dry matter yield of raya. Annals Arid Zone 31, 195-197.
Mamaril, CP, Gonzales, PB, Cacnio, VN, 1991: Sulfur management in lowland rice. In: S Portch (ed.): Proceedings of the International Symposium on the Role of Sulphur, Magnesium and Micronutrients in Balanced Plant Nutrition. The Potash and Phosphate Institute of Canada, 11-18.
Marchal, J, Pinon, A, Teisson, C, 1981: Effects of the form of potash fertilizers on the quality of pineapples in the Ivory Coast. Fruits doutre-mer 36, 737-743.
Marscher, B, Stahr, K, Renger, M, 1992: Lime effects on pine forest floor leachate chemistry and element fluxes. J. Env. Quality 21, 410-419.
Marsh, KB, Stowell, BM, Tillman, RW, 1992: Options for supplying potassium to kiwifruit vines. In: IJ Warrington, DH Greer, AM Snowball, DJ Wooley (eds): Second International Symposium on Kiwifruit, Vol I, Palmerston, 337-343.
Mengel, K, 1991: Ernährung und Stoffwechsel der Pflanze. Gustav Fischer Verlag, Stuttgart.
Mengel, K, Kirkby, EA, 1987: Principles of Plant Nutrition. International Potash Institute, Bern.
Miley, WN, Oosterhuis, DM, 1993: Effects of foliar application of five potassium fertilizers on cotton yield and quality. Res. Series Arkansas Agric. Exp. Station 425, 80-83.
Morris, RJ, 1986: The importance of sulphur in agriculture an overview. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 1-17.
Morrison, RJ, Naidu, R, Singh, U, 1987: Sulphur fertilizer requirements of Papua New Guinea and the South Pacific. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 57-66.
Mostafa, MA, El-Gala, AM, Wassif, MM, El-Maghraby, SE, 1990: Distribution of some micronutrients through a calcareous soil column under saline water application. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 263-276.
Muraka, IP, Jackson, TL, Moore, DP, 1973: Effects of N, K and Cl on N components of Russet Burbank potatoes. Agron. J. 65, 868.
Nabi, G, Rahmatullah, Salim, S, 1990: Utilization of added sulphur by groundnut on two Udic Haplustalfs. J. Indian Soc. Soil Sci. 38, 70-72.
Naik, NM, Malvi, GC, Turankar, VL, Bobde, GN, 1993: Effect of irrigation and foliar nutrition on yield attributing characters and yield of late sown chickpea. J. Soils Crops 3, 138-140.
Ni, W, 1994: Interim report on balanced fertilization of vegetable rotations in Hangzhou suburban. Zhejiang Agric. University.
Ni, W, 1995: Annual report, Zhejiang Agric. University.
Nisbet, AF, Mocanu, N, Shaw, S, 1994: Laboratory investigation into the potential effectiviness of soil-based countermeasures for soils contaminated with radiocaesium and radiostrontium. Sci. Total Env. 149, 145-154.
Nurzynski, J, Uziak, Z, Mokrzecka, E, 1980: Effects of various kinds of potassium fertilizers on the yield and quality of greenhouse tomatoes. Acta Agrobotanica 33, 197-203.
Oh, WK, 1988: Plant response to sulphur application. In: Proceedings, International Symposium on Sulphur for Korean Agriculture. Sulphur Institute, Washington, 118-130.
Omer, EA, Ibrahim, ME, Razin, AM, Ahmed, SS, 1996: Effect of spacing, nitrogen and potassium fertilization of Silybum marianum L. cultivated in newly reclaimed lands. Egyp. J. Hort. 22, 97-108.
Orlovius, K, 1996: Kalium-Menge und form bestimmen Ertrag und Qualität. Kartoffelbau 3, Sonderdruck.
Parker, MB, Gaines, TP, Gascho, GP, 1986: The chloride toxicity problem in soybean in Georgia. In: TL Jackson (ed.): Chloride and Crop Production. Potash and Phosphate Institute, Atlanta, 100-108.
Parkpian, P, Pongsakul, P, Sangtong, P, Cholitkul, W, Ratanarat, S, 1986: Indigenous soil properties influencing the availability of sulfur in selected Thai soils. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 469-487.
Pasricha, NS, Aulakh, MS, 1990: Effect of phosphorussulphur interrelationship on their availability from fertilizer and soil to soybean (Glycine max.) and linseed (Linum usitatissimum L.). In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 277-279.
Petrov-Spiridonov, NA, 1991: Effect of potassium on the development of wheat seedlings on saline soils. Moscow Univ. Soil Sci. Bul. 46, 42-44.
Prasad, M, Burge, GK, Spiers, TM, Fietje, G, 1993: Chloride-induced leaf breakdown in kiwifruit. J. Plant Nutr. 16, 999-1012.
Rabeh, MRM, Sweelam, ME, 1990: Efficiency of potassium, nematicide and their combination in relation to absorption of potassium, population of citrus nematode (Tylenchulus semipenetrans) and yield of mandarin (Citrus reticulata). Indian J. Agric. Sci. 60, 52-55.
Rader, LF, White, LM, Whittaker, CW, 1943: The salt indexa measure of the effect of fertilizers on the concentration of the soil solution. Soil Sci. 55, 201-218.
Rahman, F, Sharma, PB, Nandi, NC, 1978: Suitability of muriate of potash (KCl) and sulphate of potash (K2SO4) for foliar spray in young and mature tea. Two and a bud 25, 97-98.
Rahman, MH, Chowdhury, MKA, Hossain, AHMD, 1986: Effect of sulphur on yields and sucrose content of sugarcane in calcareous and non-calcareous soils of Bangladesh. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 254-280.
Raschke, K, 1979: Movements of stomata. In: W Haupt, ME Feinleib (eds): Encyclopedia of Plant physiology. Vol. VII, Springer Verlag, Berlin, 383-441.
Rashid, M, Bajwa, MI, Hussain, R, Naeem-ud-Din, M, Rehman, F, 1992: Rice response to sulphur in Pakistan. Sulphur in Agriculture 16, 3-5.
Rasool, G, Khattack, JK, Bhatti, A, 1987: Comparitive effect of potassium sulphate vs potassium chloride on the yield and chemical composition of maize under D. I. Khan conditions. Pakistan J. Agric. Res. 8, 29-33.
Reddy, DS, Reddy, DR, Chary, GVN, 1978: A note on the effect of sulphate of potash on hydraulic conductivity, water holding capacity and crop yields under rainfed agriculture at Anantapur. Annals of Arid Zone 17, 236-238.
Refat, A, Youssef, Chino, M, 1990: Relation between sulphate and nutrient distribution across the rhizosphere in different soils. In: Proceedings Middle East Sulphur Symposium, 12-16 February, 1990, Cairo, 229-243.
Reynolds-Vargas, JS, Richter, DD, Bornemisza, E, 1994: Environmental impacts of nitrification and nitrate adsorption in fertilized andisols in the Valle Central of Costa Rica. Soil Sci. 157, 289-299.
Robinson, SP, Downton, JS, 1984: Potassium, sodium, and chloride content of isolated intact chloroplasts in relation to ionic compartmentation in leaves. Arch. Biochem. Biophys. 228, 197-206.
Römheld, V, 1983: Ph-Veränderungen in der Rhizosphäre in Abhängigkeit vom Nährstoffangebot. Landwirtsch. Forsch. 36, 226-230.
Sachdev, MS, Chhabra, P, 1974: Transformations of S35-labelled sulfate in aerobic and flooded soil conditions. Plant and Soil 41, 335-341.
Salardini, AA, Sparrow, LA, Holloway, RJ, 1993: Effects of potassium and zinc fertilizers, gypsum and leaching on cadmium in the seed of poppies. In: NJ Barrow (ed.): Plant Nutrition From Genetic Engeneering to Field Practice: Proceedings of the Twelfth International Plant Nutrition Colloquium, 21-26 Sep. 1993, Perth. Kluwer Academic Publishers, Dordrecht, 795-798.
Salim, M, Rahmatullah, 1986: Studies on plant response to sulphur applications in Pakistan soils. In: Sulphur in Agricultural Soils. Proceedings International Symposium, Dhaka, April 20-22, Dhaka, 281-297.
Salim, M, Rahmatullah, 1987: Evaluation of sulphur status and potential for its application in Pakistan soils. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 49-56.
Samosir, SSR, 1991: Sulfur studies at Hasanuddin University. In: G Blair, R Lefroy (eds): Sulfur fertilizer policy for lowland and upland rice cropping systems in Indonesia. Austr. Center for Int. Agric. Res., Canberra, 97-100.
Sanders, D, 1984: Gradient-coupled chloride transport in plant cells. In: GA Gerencser (ed.): Chloride Transport Coupling in Biological Membranes and Epithelia. Elsevier Sci. Publ., 63-120.
Santoso, D, 1989: Phosphorus and sulfur characteristics and fertilization of upland acid soil of the humid tropics. University of New England, Armidale.
Sarkar, A, Faroda, AS, Bhatia, PC, 1991: Studies on sources of sulfur in pearlmillet-wheat sequence under semi-arid conditions. Indian J. Agron. 36, 148-152.
Satyanarayana, G, 1981: Problems of grape production around Hyderabad. AP Grape Growers Association and AP Agricultural University, Hyderabad, 66.
Saurat, A, Boulay, H, 1985: Sulphate of potash fertilizer. Potash Review 6, Subject 16, 103rd suite.
Scarsbrook, CE, 1965: Nitrogen availability. In: WV Bartholomew, FE Clark (eds): Soil Nitrogen. American Society of Agronomy, Madison, Wisconsin, 486-501.
Shah, R, 1987: Status report on the role of sulphur as a plant nutrient in Nepal. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 42-48.
Shanmugam, KS, 1995: Sulphur nutrition of sugarcane. Fertiliser News 40, 23-26.
Shin, JS, 1987: Sulphur in Korean agriculture. In: Proceedings of the Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bangkok, 26-30 January 1987. Fertilizer Advisory, Development and Information Network for Asia and the Pacific (FADINAP), Bangkok, 76-82.
Siddaramappa, SN, Shivaraj, B, Andani, G, 1993: Effect of source and levels of potassium and sulphur with molybdenum seed dressing on yield and quality of groundnut. Mysore J. Agric. Sci. 27, 124-129.
Singh, BP, Singh, HG, 1984: Comparative efficiency of sulphur on sulphur content at growth stages and uptake by mustard on vertisols. Indian J. Agron. 29, 179-184.
Singh, R, Duhan, BS, 1993: Phosphorus and sulphur nutrition of some chickpea (Cicer arietinum L.) cultivars. Crop Res. (Hisar) 6, 44-50.
Singh, V, Singh, A, Mehta, VS, 1995: Effect of sulphur sources and levels on yield and uptake of nutrients by garlic. Fertiliser News 40, 47-49.
Snoeck, D, NGoran, K, Snoeck, J, 1986: Study of the forms of potassium on the foliar chemistry of robusta coffee in the Ivory Coast. Effect of chlorine. Cafe Cacao The 30, 37-50.
So, HB, Tayler, DW, Yates, WJ, McGarity, JW, 1978: Amelioration of structurally unstable grey and brown clays. In: WW Emerson, RD Bond, AR Dexter (eds): Modification of Soil Structure, 325-333.
Sparrow, LA, Salardini, AA, Johnstone, J, 1994: Field studies of cadmium in potatoes (Solanum tuberosum L.). III. Response of cv. Russet Burbank to sources of banded potassium. Austr. J. Agric. Res. 45, 243-249.
Srivastava, HS, 1980: Regulation of nitrate reductase activity in higher plants. Phytochemistry 19, 725-733.
Stevenson, FJ, 1986: Cycles of Soil. John Wiley & Sons, New York.
Suarez-Vásquez, S, Carrillo-Pacvhón, IF, 1984: Comportamiento de tres fertilizantes potasicos en un dystrandept tipico. Cenicafé (Colombia) abril/junio, 31-39.
Sukhvinder, K, Gupta, SK, Munshi, SK, 1994: Lipid composition of groundnut (Arachis hypogaea L.) kernels a sinfluenced by sulphur application. J. Res. Punjab Agric. Univ. 31, 307-312.
Takkar, PN, 1988: Sulphur status in Indian soils. Proc. TSI-FAI Symp. Sulphur in Indian Agriculture. New Delhi, 1-31.
Tandon, HLS, 1991: Sulphur Research and Agricultural Production in India. The Sulphur Institute, Washington.
Tandon, HLS, Kemmler, G, 1986: Potassium Sulphate for Quality Crops in India. International Potash Institute, Bern.
Thier, AL, Sammons, DJ, Grybauskas, AP, Tomerlin, JR, 1986: The effect of chloride on the incidence and severity of powdery mildew in soft and red winter wheat. In: TL Jackson (ed.): Chloride and Crop Production. Potash and Phosphate Institute, Atlanta, 62-72.
Thirumalaisamy, K, Sathasivam, R, Natarajaratnam, 1986: Effect of sulphur fertilisation on nutrient content, dry matter production and yield of groundnut. Madras Agric. J. 73, 151-154.
Tisdale, SL, Nelson, WL, Beaton, JD, 1985: Soil Fertility and Fertilizers. Macmillan Publishers, New York.
Tiwari, KN, Nigam, V, Pathak, AN, 1983: Effect of sulphur fertilization on yield response and sulphur and nitrogen composition of rice grown in the soils of Kanpur district. Indian J. Agric. Sci. 53, 812-819.
Trivedi, JK, Verma, MM, 1996: Quality of rice (Oryza sativa L.) as affected by potassium sources with molybdenum. J. Soils Crops, 6, 102-104.
Tu, S, Racz, GJ, 1994: Effect of KCl on bioavailability of manganese. Can. J. Soil Sci. 74, 93-98.
Vaes, AG, 1987: Sulphur fertilizer requirements and availability in Asia. In: Symposium on Fertilizer Sulphur Requirements and Sources in Developing Countries of Asia and the Pacific, Bankok, 55-66.
van Beusichem, ML, Neeteson, JJ, 1982: Urea nutrition of young maize and sugar-beet plants with emphasis on ionic balance and vascular transport of nitrogenous compounds. Neth. J. agric. Sci. 30, 317-330.
von Braunschweig, LC, 1988: Types of K-fertilizers in the K-replacement strategy. In: Nutrient Balances and the Need for Potassium. Proceedings of the 13th IPI-Congress, Reims, August 1986, 253-262.
von Peter, A, 1981: Sulphate of potash in Asia. The British Sulphur Corporation Limited, London.
von Peter, A, Krauss, A, Maibaum, W, 1995: Potassium sulphate. In: HLS Tandon (ed.): Sulphur Fertilizers for Indian Agriculture: a Guidebook. Fertiliser Development and Consultation Organisation, New Delhi, 55-65.
von Uexküll, HR, 1986: Sulphur interactions with other plant nutrients. In: Sulphur in Agricultural Soils. Proceedings of the International Symposium, Dhaka, April 20-22, 1986, Dhaka, 212-242.
von Uexkull, HR, Sanders. JL, 1986: Chlorine in the nutrition of palm trees. In: TL Jackson (ed.): Chloride and Crop Production. Potash and Phosphate Institute, Atlanta, 84-99.
von Uexküll, HR, 1995: Population growth and food security in Asia. Int. Fert. Corresp., 36, 2-3.
Wang, M, 1987: The Effect of Chloride on Common Root Rot of Cereals. University of Sasketchewan, Saskatoon.
Warman, PR, Sampson, HG, 1994a: Effect of sulfur additions on the yield and elemental composition of canola and spring wheat. J. Plant Nutr. 17, 1817-1825.
Warman, PR, Sampson, HG, 1994b: Effect of sulfur additions on the yield and elemental composition of red clover and ryegrass. Com. Soil Sci. Plant Anal. 25, 1471-1481.
Wehrmann, J, Hähndel, R, 1984: Relationship between N- and Cl-nutrition and NO3-content of vegetables. Vith Intern. Colloquium for the Optimization of Plant Nutrition, Montpellier. Proceed. Vol. 2, 679-685.
Westermann, DT, James, DW, Tindall, TA, Hurst, RL, 1994a: Nitrogen and potassium fertilization of potatoes: sugars and starch. Am. Potato J. 71, 433-453.
Westermann, DT, Tindall, TA, James, DW, Hurst, RL, 1994b: Nitrogen and potassium fertilization of potatoes: Yield and specific gravity. Am. Potato J. 71, 417-431.
White, LM, Ross, WH, 1939: Effect of various grades of fertilizer on the salt content of the soil solution. J. Agr. Res. 59, 81-99.
Wu, X, 1995: K and Mg in balanced fertilization in Chinas Tea Gardens. Tea Research Institute, CAAS, Hangzhou, International Potash Institute, Basel.
Wu, X, Ruan, J, 1994: Backgrounds of K, Mg and S in the soil of tea gardens and prospects of the application of their fertilizer in China. In: Proceedings of the Intern seminar on Integrated Crop Management in Tea: Towards higher Productivity. International Potash Institute, Basel.
Yamauchi, M, 1989: Rice bronzing in Nigeria caused by nutrient imbalances and its control by potassium sulfate application. Plant and Soil 117, 275-286.
Yanishevskii, FV, Kuzmin, LS, Konchits, VA, Chernikov, V, 1990: Effect of prolonged application of various amounts and forms of potassium fertilizers on the content, composition, and properties of nitrogen-bearing and organic compounds in sod-podzolic soil. Soviet Soil Sci. 22, 53-61.
Zehler, E., Kreipe, H, Gething, PA, 1981: Potassium Sulphate and Potassium Chloride. IPI Research Topics No. 9. International Potash Institute, Bern.
Zhu, B, Alva, AK, 1993: Trace metal and cation transport in a sandy soil with various amendments. Soil Sci. Soc. Am. J. 57, 723-727.
Zhu, ZL, Liu, CG, Jiang, BF, 1984: Mineralization of organic nitrogen, phosphorus and sulfur in some paddy soils of China. In: Organic matter and rice. International Rice Research Institute, Los Baños.