

Storage methods for extending shelf life of fresh, edible bamboo shoots [Bambusa oldhamii (Munro)]
Kleinhenz, V.; Elsmore, S.; Harrower, K.; Lyall, T.; Gosbee, M.; Blackburn, K.; Midmore, D. J., 2000
Postharvest Biology and Technology, 19, 253-264
Postharvest Biology and Technology 19 (2000) 253264
Storage methods for extending shelf life of fresh, edible bamboo shoots [Bambusa oldhamii (Munro)]
V. Kleinhenz a *, M. Gosbee b, S. Elsmore a, T.W. Lyall a, K. Blackburn b, K. Harrower a, D.J. Midmore a
a Plant Sciences Group, Primary Industries Research Centre, Central Queensland University, Bruce Highway, North Rockhampton, Qld 4702, Australia
b Department of Primary Industry and Fisheries, GPO Box 990, Darwin, NT 0801, Australia
Received 13 November 1998; accepted 5 March 2000
Abstract
Fresh bamboo [Bambusa oldhamii (Munro)] shoots were harvested, and stored in the open or packaged in a range of materials at different temperatures (1, 2, 8, 11, 20, 25°C) to determine their shelf life under various storage conditions. Shelf life could be extended by primarily reducing storage temperature and secondarily packaging bamboo shoots. Under traditional storage at ambient temperature (20-25°C) without packaging, high transpirational weight loss (22.6 ± 0.5% of initial fresh weight after 6 days storage duration) limited shelf life of bamboo shoots to 1 day. When water loss was reduced to 2.2 ± 0.1% of initial fresh weight by enclosing shoots in low-density polyethylene (LDPE) film, shelf life was extended to 6 days. Under those conditions, respiration (2.97 ± 0.22 mmol CO2 kg-1 h-1) accounted for a larger percentage of total weight loss (35% after 6 days) and a Q10 of 2.3 for respiration between 20 and 2°C stressed the need to quickly cool bamboo shoots after harvest and reduce storage temperatures. At temperatures above 1-2°C (i.e. 8°C), discoloration and fungal growth reduced shelf life of bamboo shoots to not more than 10 days. Microbial decay appeared to be the primary cause for this deterioration in quality since bacterial, fungal and Coliform microorganisms were present on external surfaces and internal tissues of shoots. At 1-2°C, respiration (c. 0.7 mmol CO2 kg-1 h-1) was low and, therefore, transpiration accounted for up to 94% of total weight loss. Shelf life at these low storage temperatures was affected by weight loss and decline in visual packaging quality. Weight loss from packages was greater in the more permeable materials, e.g. the shelf life of shoots in macro-perforated LDPE bags (8.9% area perforated) was reduced to 17 days. In contrast, accumulation of condensate in low-permeable materials (LDPE bags and particularly heat-sealed PVC film) reduced shelf life to 21 and 14 days due to loss of visual packaging quality. The most suitable packaging materials were thin, micro-perforated (45mm, 0.01% perforation) LDPE bags and non-perforated LDPE film (10.5mm thick). These reduced total weight loss of bamboo shoots to about 5% after 28 days, minimized condensation within packages and extended shelf life to and probably beyond 28 days. © 2000 Elsevier Science B.V. All rights reserved.
Keywords: Edible bamboo shoots [Bambusa oldhamii (Munro)]; Postharvest packaging; Storage temperature; Weight loss; Respiration; Discoloration; Microbial decay
1. Introduction
Bamboo shoots are immature, expanding culms that emerge from nodes of the (pseudo-) rhizome of bamboo plants. The edible part consists of meristematic cell tissue with regions of rapid cell division and differentiation which is enveloped in protective, non-edible leaf sheaths (Farrelly, 1984). During harvest, shoots are cut below or slightly above the soil surface with a spade or hoe, leaving a severe wound at the cut end.
Although most of the approximately 1,300 identified bamboo species produce shoots suitable for human consumption, the commercially most important species belong to a few genera, e.g. Phyllostachys, Bambusa and Dendrocalamus. There are no reliable figures for world production, trade and consumption of bamboo shoots. Cusack (1999) estimated that more than 2 million t are consumed annually. China with the largest bamboo industry worldwide has a total of about 3.8 million ha bamboo forest (Li and Xu, 1997). About 130,000 t dry weight (approximately 1.3 million t fresh weight) of bamboo shoots are produced per year in China (Shi et al., 1997). Major export countries are China, Thailand and Taiwan and the major import country is Japan, importing about 133,000 t year-1 (Midmore, 1998). Canned and preserved produce currently dominates international trade but, due to increased consumer desire for non-processed food, it is projected that the share of fresh shoots will significantly increase in the future.
Studies on causes for postharvest deterioration of quality in bamboo shoots are limited. In local markets around Southeast and East Asia, bamboo shoots are usually stored and sold at ambient temperatures and unpackaged. However, for transport from production sites into urban centers or overseas, Hua (1987) emphasized the requirement for low temperatures and packaging to reduce transpiration. Liu (1992) attributed loss of quality to the high respiration rate (>4.08 mmol CO2 kg-1 h-1) at elevated storage temperature (20°C). Oshima (1931) described discoloration as a major cause of quality loss in shoots. To our knowledge, there is no study on the role of microorganisms in postharvest quality loss and decay of bamboo shoots. However, Wang and He (1989) reported that addition of a fungicide to bamboo shoots packaged in polyethylene film extended their shelf life to 62 days at 0°C. These and very similar results from Liao (1989) point to the significance of microbial action during postharvest storage of fresh bamboo shoots.
This study was conducted to determine the effect of storage temperatures and several packaging materials on (i) weight loss, (ii) respiration, (iii) discoloration and fungal infection, and (iv) shelf life of fresh bamboo shoots. An examination (v) of microbial load on external and internal tissues of bamboo shoots was included to provide evidence for microbial deterioration of quality of stored bamboo shoots.
2. Materials and methods
2.1. Plant materials
Fresh shoots of Bambusa oldhamii (Munro) were harvested in January 1998 and February 1999 from a commercial bamboo farm ("Bamboo Australia") at Belli Park near Eumundi, Queensland, and in March 1998 from an experimental area at the Coastal Plains Horticultural Research Station of the Department of Primary Industry and Fisheries of the Northern Territory (DPIF-NT) near Darwin, Northern Territory (Australia). Shoots were cut about 5-10 cm below the soil surface with a spade. In 1998, shoots from Belli Park were transported to Central Queensland University (CQU) in Rockhampton over night by airmail and in 1999 on ice by car. In Darwin, shoots were transported on ice to a laboratory building of DPIF-NT. Before assigning to treatments, 1 cm was removed from the cut end of the shoots with a sharp kitchen knife. The surface of all bamboo shoots was dried with paper towels. Weight of shoots from Belli Park averaged 124 ± 9.7 g shoot-1 (1998) and 148 ± 8.6 g shoot-1 (1999), and from Darwin 213 ± 28.9 g shoot-1. There were no significant differences between treatments in those initial fresh shoot weights.
2.2. Packaging and storage temperatures
During the three experiments, the following packaging materials (NationalPack Pty Ltd and Gelpack Enterprises Pty Ltd, Australia) were tested for bamboo shoots:
· Open storage (control)
· Low-density polyethylene (LDPE) film (10.5 mm thick)
· Macro-perforated LDPE bags (45 mm thick, 8.94% area perforated)
· Micro-perforated LDPE bags (35 mm thick, 0.01% area perforated)
· LDPE bags (45 mm thick)
· Heat-sealed, food grade polyvinyl chloride (PVC) film (90 mm thick).
Only subsets of these packaging treatments were compared in the three independent experiments (Table 1). In the control, individual bamboo shoots were placed in open cardboard boxes at CQU and in open plastic boxes at DPIF-NT. For the packaging treatments, one layer of LDPE film was firmly wrapped around individual bamboo shoots at CQU. At DPIF-NT, plastic boxes were enclosed with LDPE film. In the treatments with LDPE bags, shoots were placed individually in clip-top bags (23 cm ΄ 33 cm) which were press-sealed. The micro-perforated LDPE bags were sealed with a firm knot and the PVC film was heat-sealed close to shoots and excess film material cut off.
|
Table 1 Summary of treatments of storage temperature and packaging material for each of three experiments |
||||
|
Year |
Location |
Storage temperature |
Replications |
Packaging material |
|
1998 |
CQU |
3 |
macro-perforated LDPE bag |
|
|
|
|
25°C |
3 |
macro-perforated LDPE bag |
|
|
DPIF-NT |
2°C |
6 |
open, LDPE film |
|
|
|
20°C |
6 |
open, LDPE film |
|
1999 |
CQU |
1°C |
3 |
open, LDPE film, macro-perforated LDPE bag, micro-perforated LDPE bag, LDPE bag, heat-sealed PVC |
|
|
|
8°C |
3 |
macro-perforated LDPE bag, LDPE bag |
Subsets of the packaging treatments were exposed to six different storage temperatures (1, 2, 8, 11, 20 and 25°C; Table 1). Relative humidity in the storage facilities was >95% at 1°C, >80% at 2, 8 and 11°C, >70% at 20°C and >60% at 25°C. Experiment duration at CQU was 28 days in 1998 and 30 days in 1999, and 6 days at DPIF-NT. All treatments at CQU were replicated thrice and those at DPIF-NT six times.
2.3. Weight loss
In the trials at CQU, shoot weight was determined destructively (i.e. shoots were not put back into storage) after 10, 20 and 30 days in 1998 and after 10 and 28 days storage time in 1999. At DPIF-NT, shoot weight was recorded non-destructively after 1, 2, 4 and 6 days. In the trials at CQU in 1999, not only the weight of bamboo shoots but also the weight of packaging units (i.e. bamboo shoot and packaging material) was recorded non-destructively immediately after harvest and 2, 4, 6, 10, 14, 21 and 28 days in storage (1°C). Weight loss was determined as the percentage of the initial fresh shoot weight remaining after storage. The weight of water condensed in packages was calculated as the difference between fresh weight loss of bamboo shoots and weight loss from packages. Water content of bamboo shoots was determined as the difference between fresh weight and the (dry) weight of shoots after dicing and drying for 48 h at 70°C.
2.4. Respiration
Respiration rates were determined at DPIF-NT for bamboo shoots stored at 2°C and 20°C in open plastic boxes and in plastic boxes enclosed with LDPE film. Measurements were made before storage and after 1, 2, 4 and 6 days storage. Six replications of each treatment were removed from storage and sealed in an airtight container. A 1-ml gas sample of the head space was taken at 0 and 1 h incubation at the corresponding temperatures. The gas samples were injected into a gas chromatograph (Gow Mac Series 550, USA) fitted with a thermal conductivity detector and a column packed with Poropak Q (100/200, Supelco, Inc., USA). Operating conditions of the gas chromatograph were: oven temperature 65°C, detector temperature 100°C, injection port temperature 85°C and carrier gas (helium) 4 ml min-1 flow rate. The response of the gas chromatograph was standardized with a mixture containing 0.564% ± 0.001% CO2 in air and the respiration rate calculated accordingly. Weight loss by respiration after 6 days storage period was calculated taking glucose as the primary respiratory substrate and assuming constant evolution of CO2 at the average of measured respiration rates. Weight loss by transpiration was calculated by subtracting respiratory weight loss from the measured total weight loss. The temperature coefficient Q10 for respiration between 20 and 2°C was calculated as
![]()
where k1 and k2 are the respiration rates at 2 and 20°C, respectively (Larcher, 1995).
2.5. Discoloration and fungal infection
External discoloration and fungal infection was visually assessed (3 replications) for bamboo shoots stored at 1°C in the various packaging materials and at 8°C in LDPE bags after 2, 4, 7, 10, 14, 21 and 28 days storage period in the experiment at CQU in 1999. Discoloration was rated on a 5-point scale (where 0 = bleached white surface color, 1 = some yellow stain, 2 = some dark spots, 3 = moderately discolored and 4 = highly discolored). Fungal infection was rated as the percentage of surface of shoots covered with fungal mycelia: 0 = 0% to 4 = 100% surface cover.
2.6. Qualitative and quantitative microbial load
Eighteen fresh bamboo shoots were sampled after harvest at CQU in 1998 to qualitatively evaluate microbial load on surfaces of external leaf sheaths and cut ends, and quantitatively evaluate microbial load in the internal tissues. Sampling of shoots was performed under aseptic conditions, i.e. all tools and containers were sterilized with hypochlorite solution and ethanol between sampling of individual shoots.
A 2-cm2 area on the surface of external leaf sheaths and the cut end of each bamboo shoot were swabbed with sterile cotton swabs moistened with sterile distilled water using the standard method outlined in Jay (1996). Swabs were streaked on growth media prepared for bacterial, mycological and coliform counts. Nutrient agar (NA) was the growth media for the general bacterial count, half-strength potato dextrose agar (hPDA) for the general mycological count and chromocult agar (Chcult) for the total coliform count. All plates, replicated three times for each assay, were incubated for 3 days at 28° C. Qualitative microbial loads on the external surfaces of bamboo shoots were visually assessed as aerobic plate counts. The relative amount of growth was rated on a 8-point scale: 0 = nil to 7 = prolific (James, 1974).
For quantification of microbial load in internal tissues, the outer leaf sheaths of six bamboo shoots were removed to the first leaf-bearing basal node. These shoots were surface-sterilized with 1% silver nitrate (1 min) and 5% sodium chloride solution (30 sec) and then washed with sterile distilled water. Three disk-shaped segments were cut from each shoot with a sterile scalpel where the diameter was approximately 10 cm (about 5-15 cm distance from the cut end). The three segments of each shoot were then systematically assigned to bacterial, mycological and coliform counts. The segments were aseptically transferred onto the surface of prepared media plates (6 replications). After incubation for two days at 28°C, quantitative microbial load was determined as number of colony-forming units (cfu) per unit area.
2.7. Data analysis
All storage trials were laid out as single factor experiments. A summary of treatments and replications is presented in Table 1. Only differences between packaging materials at CQU (1999) were analyzed with ANOVA and means separated with the LSD test (p = 0.05). All other differences were compared with standard errors (S.E.). Data were analyzed with SAS for Windows Version 6.12 (SAS Institute Inc., 1993).
3. Results
3.1. Weight loss as affected by storage temperature
Generally, shoots which had lost more than 5% of their initial fresh weight were visually rated unacceptable. When weight loss progressed beyond this stage, the cut ends of shoots desiccated and cracks appeared. Subsequently, the lateral surface became pale and shriveled.
Measurements of weight loss of bamboo shoots at different storage temperatures were confounded to some extent by differences in relative humidity and vapor pressure gradients between shoots and air. Weight loss of bamboo shoots in macro-perforated LDPE bags was accelerated by higher storage temperatures (Fig. 1). The linear shape of curves made it possible to extrapolate shelf life of bamboo shoots based on a critical weight loss of 5%: at 1, 8, 11 and 25°C, shoots lost 5% of their weight within approximately 17, 10, 5 and 1 day(s), and these periods corresponded to their shelf lives. All differences in weight loss between storage temperatures were significant as indicated by standard errors.
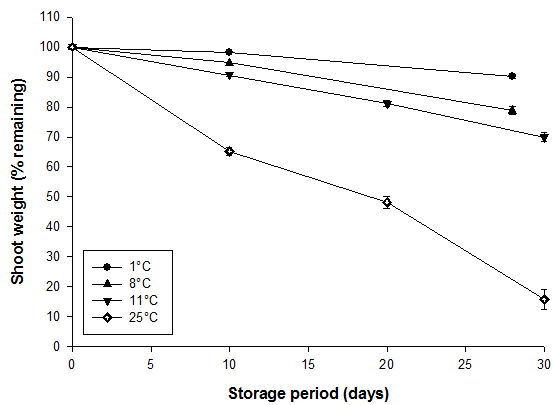
Fig. 1. Weight loss of bamboo shoots (B. oldhamii) stored in macro-perforated LDPE bags as affected by storage temperature. Vertical bars represent S.E. (n = 3) and are not shown when the values are smaller than the symbol.
3.2. Weight loss as affected by packaging materials
Weight loss from packages reflected the permeability of the five different materials at 1°C (Fig. 2). After 6 days of storage, weight loss of shoots in macro-perforated LDPE bags was significantly greater than in all other materials. After 22 days, weight loss of shoots in micro-perforated LDPE bags and LDPE film exceeded that of shoots in LDPE bags and heat-sealed PVC. Therefore, packaging materials could be categorized into three groups: low-permeable materials (heat-sealed PVC film and LDPE bags), medium-permeable materials (LDPE film and micro-perforated LDPE bags), and highly permeable material (macro-perforated LDPE bags).
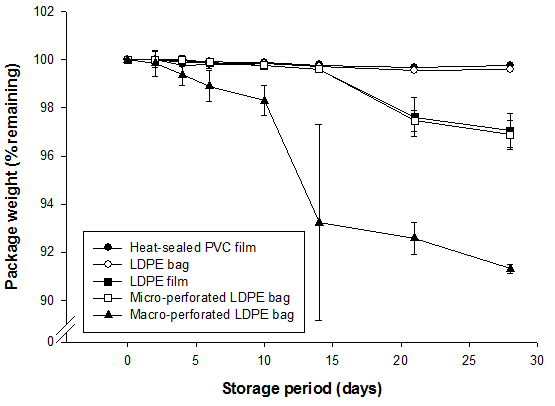
Fig. 2. Weight loss of packages of bamboo shoots (B. oldhamii) stored at 1°C as affected by packaging materials. Vertical bars represent S.E. (n = 3) and are not shown when the values are smaller than the symbol.
The experiment included a control treatment with shoots stored in the open. Even at the lowest storage temperature (1°C), bamboo shoots stored in the open lost 7.67% weight within 10 days (Table 2) and their shelf life of was no more than 7 days (Table 5). Packaging in any material significantly reduced this weight loss.
After 10 days of storage, there were no statistically significant differences in total weight loss of bamboo shoots between packaging materials (Table 2). However, after 28 days, weight loss of shoots was much greater in macro-perforated LDPE bags than in the less permeable materials. Weight loss was disproportionately greater at 28 days compared to 10 days. Although we did only sample shoots after 10 and 28 days storage period, weight loss of bamboo shoots might have followed a sigmoid relationship with small weight loss during the initial storage period, rapid loss thereafter and finally only slight weight loss when shoots become highly desiccated.
|
Table 2 Influence of packaging material on total weight loss (% of initial fresh weight) of bamboo shoots (B. oldhamii) stored at 1°C after different storage periods |
||
|
Packaging material |
Storage perioda |
|
|
|
10 days |
28 days |
|
Control (open storage) |
7.67a |
26.96a |
|
Macro-perforated LDPE bag |
1.80b |
9.85b |
|
Micro-perforated LDPE bag |
0.22b |
4.97c |
|
LDPE film |
0.37b |
5.58c |
|
LDPE bag |
0.09b |
4.17c |
|
Heat-sealed PVC film |
1.59b |
5.96c |
|
a Means separation by LSD test (p = 0.05, n = 3): means in each column followed by the same letter are not significantly different |
||
There was an inverse relationship between weight of condensate in packages and weight loss from packages (Table 3). In the less permeable materials (particularly LDPE bags and heat sealed PVC film) there was more condensate in packages but less weight loss from packages. The opposite was true for more permeable materials (particularly macro-perforated LDPE bags). Both condensation and weight loss from packages reduced shelf life of bamboo shoots. Due to excessive loss from packages (Table 3), total weight loss of bamboo shoots in highly permeable macro-perforated LDPE bags was significantly greater than in all other materials (Table 2), reducing their shelf life to 17 days (Table 5). In contrast, condensation in low-permeable LDPE bags and particularly heat-sealed PVC film reduced shelf life of bamboo shoots to 21 and 14 days due to deterioration of visual quality of packages. Shelf life in the medium-permeable materials (micro-perforated LDPE bags and LDPE film) extended to and possibly beyond the 4-week storage period.
|
Table 3 Influence of packaging material on condensate in packages and weight loss from packages (% of initial fresh weight) of bamboo shoots (B. oldhamii) stored for 28 days at 1°C |
||
|
Packaging material |
Condensate in packagea |
Weight loss from packagea |
|
Macro-perforated LDPE bag |
0.62c |
9.23a |
|
Micro-perforated LDPE bag |
1.56bc |
3.41b |
|
LDPE film |
2.57bc |
3.01b |
|
LDPE bag |
3.74ab |
0.43c |
|
Heat-sealed PVC film |
5.69a |
0.27c |
|
a Means separation by LSD test (p = 0.05, n = 3): means in each column followed by the same letter are not significantly different |
||
3.3. Respiration
Respiration rates of bamboo shoots were strongly affected by storage temperature (Fig. 3). Except for immediately after harvest when temperature treatments had barely been imposed, respiration was significantly greater in bamboo shoots stored at 20°C than at 2°C, irrespective of packaging. At 2°C, the respiration rate of shoots was about 1.52 mmol CO2 kg-1 h-1 after harvest and declined significantly to reach an equilibrium of 0.45 mmol CO2 kg-1 h-1. At 20°C, there was an immediate increase in respiration rate at 1 day reaching 3.84 and 5.80 mmol CO2 kg-1 h-1 for shoots enclosed with LDPE film and shoots stored in the open, respectively. These peaks were followed by a rapid decrease on day 2 and a slow decrease thereafter. After 6 days, bamboo shoots which were stored in the open lost 23.5 ± 0.6% of their total weight at 20°C (Table 4). Average respiration rates differed between storage temperatures but not between packaging treatments (Table 4). Transpiration was greater at the higher storage temperature and when shoots were stored in the open. When transpiration was reduced by packaging shoots, respiration accounted for a larger percentage of total weight loss, i.e. 35% at 20°C (Table 4). The Q10 for respiration between 20 and 2°C was 2.0 for shoots stored in the open and 2.3 for shoots enclosed with LDPE film.
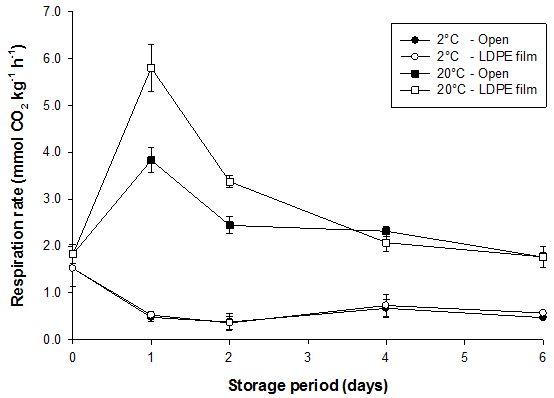
Fig. 3. Respiration rate of bamboo shoots (B. oldhamii) as affected by storage temperature and packaging. Vertical bars represent S.E. (n = 6) and are not shown when the values are smaller than the symbol.
|
Table 4 Influence of storage temperature and packaging on weight loss of bamboo shoots (B. oldhamii) by respiration and transpiration (mean ± S.E.) after 6 days storage |
|
||||||
|
Storage |
Average |
Weight loss (% of initial fresh weight) |
Transpiration/ |
|
|||
|
conditions |
respiration rate |
Respirationa |
Transpiration |
Total |
total |
||
|
|
(mmol CO2 kg-1 h-1) |
|
(%) |
|
|||
|
2°C |
|
|
|
|
|
||
|
Open |
0.71 ± 0.18 |
0.26 ± 0.07 |
4.4 ± 0.2 |
4.7 ± 0.3 |
94 ± 2 |
||
|
LDPE film |
0.67 ± 0.17 |
0.27 ± 0.06 |
1.3 ± 0.3 |
1.5 ± 0.4 |
87 ± 3 |
||
|
20°C |
|
|
|
|
|
||
|
Open |
2.44 ± 0.19 |
0.96 ± 0.08 |
22.6 ± 0.5 |
23.5 ± 0.6 |
96 ± 1 |
||
|
LDPE film |
2.97 ± 0.22 |
1.17 ± 0.09 |
2.2 ± 0.1 |
3.4 ± 0.1 |
65 ± 2 |
||
|
a taking glucose as the respiratory substrate, n = 6 |
|
||||||
3.4. Discoloration and fungal infection
The highest scores (i.e. the least quality) for discoloration and fungal infection of bamboo shoots were recorded at the higher storage temperature (Fig. 4). After 6 days of storage, brownish-black spots appeared on shoots stored at 8°C and developed into dark, soft patches. After 4 weeks, these shoots were completely discolored whereas shoots at 1°C had only few yellow stains. There was no apparent fungal growth on bamboo shoots stored at 1°C throughout the storage period. However, starting 1 week after the first signs of discoloration on shoots at 8°C, fungal mycelia rapidly developed and covered the entire surface of some shoots after 4 weeks (Fig. 4). Based upon discoloration and fungal infection, shelf life of bamboo shoots stored at 1°C was possible for more than 28 days, and for those stored at 8°C for less than 10 days. The influence of packaging materials (assessed only at 1°C) was not significant (data not shown).
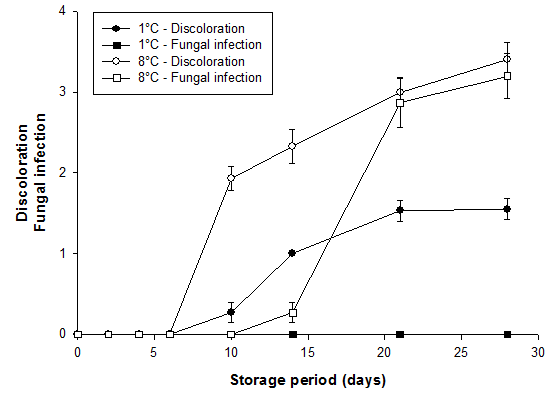
Fig. 4. External discoloration and fungal infection of bamboo shoots (B. oldhamii) stored in LDPE bags at 1°C. Vertical bars represent S.E. (n = 3) and are not shown when the values are smaller than the symbol. The higher qualitative indices for discoloration indicate greater discoloration and those for fungal infection percentage of surface cover with mycelia.
3.5. Shelf life
When considering all measured quality parameters (i.e., weight loss, discoloration, fungal infection and condensation), shelf life of bamboo shoots was possible for no more than 7 days at storage temperatures above 8°C (Table 5). At lower temperatures, packaging extended shelf life beyond the 6 days possible without packaging. The optimum packaging units at 1°C were the semi-permeable materials (micro-perforated LDPE bag and LDPE film) in which shelf life could be extended to and possibly beyond 28 days. Condensation reduced shelf life of shoots in the low-permeable materials (LDPE bag and heat-sealed PVC film) due to the accumulation of condensate in the packages, and weight loss limited the shelf life of shoots in macro-perforated LDPE bags.
|
Table 5 Influence of packaging material and storage temperature on shelf lifea (days) of bamboo (B. oldhamii) shoots (data collated from all experiments) |
||||||
|
Packaging material |
Storage temperature |
|||||
|
|
1°C |
2°C |
8°C |
11°C |
20°C |
25°C |
|
Control (open storage) |
7 |
6 |
b |
|
1.5 |
|
|
Macro-perforated LDPE bag |
17 |
|
6-10 |
5 |
|
1 |
|
Micro-perforated LDPE bag |
28 |
|
|
|
|
|
|
LDPE film |
28 |
20 |
|
|
6 |
|
|
LDPE bag |
21 |
|
6-10 |
|
|
|
|
Heat-sealed PVC film |
14 |
|
|
|
|
|
|
a Values indicate the maximum shelf life considering weight loss, discoloration, fungal infection and condensation within packages b not assessed |
||||||
3.6. Qualitative and quantitative microbial load
Both leaf sheaths and cut ends of bamboo shoots were contaminated with bacteria, fungi and coliforms (Table 6). The qualitative assessment of general bacterial, general fungi and total coliform microorganisms at cut ends yielded significantly higher scores than on leaf sheaths. Quantitative figures of microbial load in the internal tissue of bamboo shoots are presented in Table 7.
Table 6 Qualitative microbial load assessed as aerobic plate counts (counts cm-2) on surfaces of external leaf sheaths and cut ends of bamboo shoots (B. oldhamii) |
|||
|
Shoot part |
General bacterial count |
General mycological count |
Total coliform count |
|
Leaf sheath |
10.1 ± 0.55a |
9.39 ± 0.546 |
8.61 ± 0.887 |
|
Cut end |
13.8 ± 0.15 |
12.09 ± 0.634 |
12.91 ± 0.544 |
|
a mean ± S.E., n = 18 |
|||
|
Table 7 Quantitative microbial loada (cfu cm-2) in the internal tissue of bamboo shoots (B. oldhamii) |
||
|
General bacterial count |
General mycological count |
Total coliform count |
|
9.33΄10-2 ± 0.212΄10-2 |
4.66΄10-2 ± 0.424΄10-2 |
2.50΄10-1 ± 0.557΄10-1 |
|
a mean ± S.E., n = 6 |
||
4. Discussion
In Southeast and East Asia, fresh bamboo shoots are traditionally marketed at ambient temperatures and unpackaged. When temperature cannot be controlled, shelf life of shoots is primarily restricted by transpirational weight loss and is no longer than 1 day if measures for increasing humidity (e.g. baskets lined with leaves) are not undertaken. In Thailand, bamboo growers commonly sell some of their produce on local markets on a daily basis after harvesting shoots in the early mornings (Thammincha, 1988). In Taiwan, farm-gate prices drop by more than 50% for shoots not marketed on the same day of harvest (Midmore, personal communication).
Hua (1987) pointed to the importance of packaging when bamboo shoots are transported from the production sites over longer distances to the larger fresh markets in urban centers. Our own observations confirm that sealed polyethylene materials are in fact used to reduce transpirational weight loss in fresh bamboo shoots under ambient temperature conditions, e.g. on the fresh vegetable markets in Bangkok (Midmore, personal communication). In our studies, packaging in LDPE film at 20°C significantly reduced weight loss of bamboo shoots (Table 4) and extended shelf life to 6 days (Table 5).
At 20°C, respiration rates of shoots averaged 3.84-5.80 mmol CO2 kg-1 h-1 1 day after harvest (Fig. 3), and enclosed with LDPE film, respiratory weight loss accounted for an estimated 34% of total weight loss (Table 4). At this temperature, respiration peaked 1 day after harvest to decrease gradually thereafter. On this day, respiration rates were as high as those previously reported (>4.08 mmol CO2 kg-1 h-1; Liu, 1992). Although much sooner after harvest, increases in respiration have been observed in asparagus: Lill et al. (1990) measured an immediate increase in respiration rate of asparagus after harvest and Hennion and Hartmann (1990) recorded a slight increase in respiration during the first hours following harvest. This has been attributed to rapid cell expansion in asparagus as a respond to wounding (Harikrishna et al. 1991). The high respiration rates may also reflect that shoots were transported to the lab on ice, resulting in an "overshoot" of respiration. Not surprisingly, respiration was dramatically reduced by lowering temperature to 2°C. Respiration rates of bamboo shoots at 2°C resembled the pattern of decreasing respiration rates after harvest for non-climacteric produce (Fig. 3).
Although confounded with differences in relative humidity and vapor pressure deficit between shoots and air at the different temperatures, respiration accounted for a larger percentage of weight loss when water loss was reduced by enclosing bamboo shoots in LDPE film (Table 4). The temperature coefficient Q10 for respiration between 20 and 2°C was 2.0 for shoots stored in the open but 2.3 for shoots enclosed with LDPE film. Therefore, reduction in storage temperature was more crucial in reducing respiration and weight loss in bamboo shoots when they were packaged.
At storage temperatures above 1-2°C (i.e. 8°C), bamboo shoots discolored and were visually unacceptable after 6-10 days (Fig. 4, Table 5). Chen et al. (1989) attributed discoloration of bamboo shoots in their experiments to enzymatic browning caused by phenylalanine ammonia-lyase (PAL) and peroxidase (PO), activated by tissue injury at harvest. In our studies, first signs of fungal growth were detected 4 days after the first brownish-black spots appeared on shoots stored at that temperature. Fungal mycelia covered those shoots almost completely after 28 days. We believe that the browning/discoloration in our experiments was primarily caused by microorganisms since our microbiological studies revealed that bacteria, fungi and coliforms were not only present on external surfaces of bamboo shoots but also in internal tissues. It is clear that external surfaces are contaminated with microorganisms while bamboo shoots pass through the soil during growth. During harvest, microorganisms in the environment and on the harvesting tool may be drawn into the xylem, resulting in growth of rots and moulds in internal tissues. Murphy et al. 1991 isolated several fungal species in cell walls of mature bamboo culms, implying that fresh bamboo shoots may also be affected since they are themselves immature culms. The studies by Wang and He (1989) indicate that shoots are in fact largely affected by fungal microorganisms since addition of a fungicide dramatically extended their shelf life. Although bacterial, fungal and coliform microorganisms were present in the edible part of bamboo shoots, we are not able to judge whether the microbial load is great enough to be a concern when assessing food safety issues, given the present lack of food safety standards. It is unlikely to be worse than for other vegetables with exposed cut surface and edible parts close to the soil surface, many of which are eaten raw (e.g. lettuce).
At the lowest storage temperature of 1°C, discoloration and fungal growth was essentially eliminated in bamboo shoots. Only a few yellow stains developed and no fungal growth was visible after 28 days (Fig. 4). At this temperature, the choice of packaging material was a further consideration to improve postharvest quality and extend shelf life of bamboo shoots. Highly permeable macro-perforated LDPE bags (8.94% area perforated) resulted in great weight loss from packages (Table 3), reducing shelf life of bamboo shoots to 17 days (Table 5). In contrast, condensate accumulated to unacceptable levels in the low-permeable materials (LDPE bags and heat-sealed PVC film), reducing shelf life of bamboo shoots to 21 and 14 days due to loss of visual package quality.
Apart from their low permeability, film thickness of LDPE bags (45 mm) and heat-sealed PVC film (90 mm) might have played a significant role in triggering condensation in those materials. After harvest, bamboo shoots contained 93.0 ± 0.25% water and although we did not measure transpiration coefficients, we expect these to be rather high compared with other (bulky) horticultural produce (Ben-Yehoshua, 1987). Even small fluctuations in storage temperature can decrease the temperature of the humid air in packages below its dew-point temperature and consequently, water vapor condenses. In contrast to the voluminous bamboo shoots, the packaging materials have much greater heat conductivity, i.e. their temperature changes relatively quickly with changes in air temperature. Small changes in storage temperature apparently caused significant condensation of water vapor on the inner surface of the thicker materials when their temperature decreased below the dew point temperature of the air inside packages. It could also be argued that due to lower flexibility, the thicker materials were to a lesser degree in intimate contact with bamboo shoots and, consequently, there were greater temperature fluctuations which accelerated condensation. It follows that under low storage temperature, thin packaging material should be used. Bags should be of a size to fit bamboo shoots snuggly, to bring the material in intimate contact with the produce. The most suitable packaging materials in our studies were the thin, micro-perforated (45mm, 0.01% perforation) LDPE bags and the non-perforated but probably imperfectly sealed LDPE film (10.5mm thick). These materials reduced total weight loss of shoots to about 5% after 28 days, minimized condensation within packages and extended shelf life of bamboo shoots to and probably beyond 28 days.
Acknowledgements
This work was in part financially supported by the Rural Industries Research and Development Corporation (RIRDC), Barton, ACT, Australia (Research Grant UCQ-9A).
References
Ben-Yehoshua, S., 1987. Transpiration, water stress, and gas exchange. In: Weichmann, J. (Ed.), Postharvest Physiology of Vegetables. Marcel Dekker, New York, pp. 113-170.
Chen, R.Y., Liu, M.S., Chang, T.C., Tsai, M.J., 1989. Postharvest handling and storage of bamboo shoots (Bambusa oldhamii Munro). Acta Hortic. 258, 309-316.
Cusack, V., 1999. Australia's burgeoning bamboo industry. Access to Asian Foods 5, 3-4.
Farrelly, D., 1984. The Book of Bamboo. Sierra Club Books, San Francisco.
Harikrishna, K., Paul, E., Darby, R., Draper, J., 1991. Wound response in mechanically isolated asparagus mesophyll cells: a modelmonocotyledon system. J. Exp. Bot. 42, 791-799.
Hennion, S., Hartmann, C., 1990. Respiration and ethylene in harvested asparagus spears during aging at 20°C. Sci. Hortic. (Amst.) 43, 189-196.
Hua, X., 1987. Bamboo shoot cultivation and management in Japan. Symposium on Bamboo Professional Commission of Zhaihiang Forestry Society, 16.
James, W.C., 1974. A manual of assessment keys for plant diseases. Canada Department of Agriculture Publications No 1458, C.D.A., Ottawa, Canada.
Jay, J.M., 1996. Modern Food Microbiology, 5th Edition. Chapman and Hall, New York.
Larcher, W., 1995. Physiological Plant Ecology, Third Edition. Springer Verlag, berlin.
Li, S.D., Xu, C.D., 1997. The history of Chinese bamboo industry and the challenge for development in 21st century. In: Fu, M.Y., Lou, Y.P. (Eds), International Bamboo Workshop, Bamboo Towards 21st Century, 7-11 September, 1997, Anji, Zhejiang, China. The Research Institute of Subtropical Forestry, The Chinese Academy of Forestry, Anji, China, p. 4.
Liao, G.L., 1989. New packaging of fresh bamboo shoots. Bamboo Res. 1, 31-33 (in Chinese with English abstract).
Lill, R.E., King, G.A., O'Donoghue, E.M., 1990. Physiological changes in asparagus spears immediately after harvest. Scientia Hortic. 44, 191-199.
Liu, M.S., 1992. Bamboo shoot. In: Hui, Y.H. (Ed.), Encyclopedia of Food Science and Technology, Vol. 3. Wiley, New York, pp. 177-180.
Midmore, D.J., 1998. Culinary bamboo shoots. In: Hyde, K. (Ed.), The New Rural Industries, A Handbook for Farmers and Investors. RIRDC, Barton, Australia, pp. 188-195.
Murphy, R.J., Alvin, K.L., Tan, Y.F., 1991. Development of soft rot decay in the bamboo Sinobambusa tootsik. IAWA Bulletin 12, 85-94.
Oshima, J., 1931. The culture of Moso-bamboo in Japan, Part I. J. Am. Bamboo Soc. 1, 2-28.
SAS Institute Inc., 1993. SAS/STAT User's Guide, Version 6, Fourth Edition, Volumes 1 and 2, SAS Institute Inc., Cary, U.S.A.
Shi, K.S., Li, Z.Y., Lin, F.M., Zheng, R., 1997. China's country report on forestry. Asia-Pacific Forestry Sector Outlook Study, Working Paper No: APFSOS/WP/14. Forestry Policy and Planning Devision, Rome, Regional Office for Asia and the Pacific, Bangkok. FAO, Rome, Italy.
Thammincha, S., 1988. Some aspects of bamboo production and marketing. In: Rao, I.V.R., Gnanaharan, R., Sastry, C.B. (Eds), Bamboos, Current Research. Proceedings of the International Bamboo Workshop, Cochin, India, 14-18 Nov. 1988, Kerala Forest Research Institute, Kerala, India, pp. 320-327.
Wang, K.H., He, Y.F., 1989. Study on physiological characters of bamboo shoots in storage. J. Zhejiang For. Sci. Tech. 5, 19-25 (in Chinese with English abstract).