

Seasonal effects of soil moisture on soil N availability, crop N status, and yield of vegetables in a tropical, rice-based lowland
Kleinhenz, V.; Schnitzler, W. H.; Midmore, D. J., 1997
Tropenlandwirt, 98, 25-42
Der Tropenlandwirt, Beiträge zur tropischen Landwirtschaft und Veterinärmedizin, 98. Jahrgang, April 97, S. 25 - 42.
Seasonal effects of soil moisture on soil N availability, crop N status, and yield of vegetables in a tropical, rice-based lowland
V. Kleinhenz*, W. H. Schnitzler** and D. J. Midmore***
* V. Kleinhenz, Asian Vegetable Research and Development Center (AVRDC), PO Box 42, Shanhua, Tainan, Taiwan-741, ROC. Present address: Lehrstuhl für Gemüsebau, Technische Universität München, D-85350 Freising
**W. H. Schnitzler, Lehrstuhl für Gemüsebau, Technische Universität München, D-85350 Freising
*** D. J. Midmore, Asian Vegetable Research and Development Center (AVRDC), PO Box 42, Shanhua, Tainan, Taiwan-741, ROC. Present address: Biology Department, Central Queensland University, Rockhampton, Qld 4702, Australia
Abstract
The influence of seasonal variations in soil moisture on soil nitrogen availability, crop nitrogen status, root length distribution, and yield was studied in four vegetable crops year-round from 1994 to 1995 at the Asian Vegetable Research and Development Center (AVRDC) in tropical southern Taiwan. Treatments included two cultivation systems (traditional flat beds and permanent high beds) and N-fertilization methods (recommended rate and Nmin-method). Nitrification of ammonium fertilizer (ammonium sulfate) was investigated in different seasons.
Soil nitrate followed a seasonal pattern of accumulation during the dry season and low contents during the wet season. Alleviation of water stress by high beds appeared as a prerequisite for effective absorption of mineralized soil nitrogen by vegetables in the rainy season. On more flood-prone flat beds, N-absorption of vegetables was ineffective in the wet season, and more NO3 was leached below the root zone. Nitrification of ammonium fertilizer was delayed and nitrate disappeared quickly after rainfall.
Soil nitrate accumulated in the dry season when evaporation exceeded precipitation. Soil-organic-matter content was low and lack of leaching alone could not explain this accumulation. Release of clay-fixed nitrogen may play a significant role. Therefore, fertilizer rates can be reduced in this season. Vegetables with a high capacity to absorb nitrogen should be grown at the end of the dry season because nitrate is subject to quick loss with the onset of seasonal rainfall. Overall, stresses caused by excessive soil moisture in the rainy season and deficient soil moisture in the dry season were apparently more detrimental to vegetable growth than was limited availability of soil nitrogen. These results are directly applicable to lowland vegetable production in Southeast Asia.
1 Introduction
Paddy rice fields are commonly rotated with upland crops in Asia to intensify year-round vegetable production particularly in peri-urban areas around big cities. Long-term wet plowing (puddling) has destroyed soil structure and this can harm susceptible vegetable species by close successions of overwet or overdry conditions through restricted water movement (Ishii, 1986). Besides the direct impact of soil moisture, it also exerts a strong effect on the availability of nitrogen (Miller and Johnson, 1964).
Mineralization of soil organic nitrogen was found to proceed most rapidly at low soil moisture tensions of 3 to 10 kPa in some soils (Stanford and Epstein, 1974). In flooded soils, the resulting ammonium nitrogen will accumulate because of the lack of oxygen for nitrification. However under drier upland conditions, NH4 is usually quickly oxidized to nitrate (NO3) which can accumulate at substantial levels (Terry and Tate, 1980).
Availability of soil nitrogen to upland crops is limited by various processes: (1) leaching of the highly mobile NO3-ion (Koch, 1987), (2) denitrification of nitrate to N2O and N2 (Patrick and Wyatt, 1964), (3) leaching of ammonium (Harmsen and Kolenbrander, 1965), (4) immobilization of ammonium-N by microorganisms (Sowden, 1976), and (5) fixation of ammonium to clay minerals (Allison et al., 1953). The latter process describes the ability of 2:1 clay minerals such as illite and vermiculite to entrap nonexchangeable ammonium ions between their silicate sheets. Conditions that might adversely affect the biological process of nitrification of ammonium are excessive moisture, high temperatures, and soil properties (Justice and Smith, 1962).
Rice can absorb NH4-N more effectively than dicotyledons since roots of graminaceous plants show comparatively low values of cation exchange capacity and are, therefore, effective in absorbing monovalent cations (Nõmmik, 1965). Cultivation under anaerobic (flooded) soil conditions prevents oxidation of ammonium to nitrate. In contrast, roots of upland plants are less effective in using NH4 and absorb NO3 considerably more rapidly (Scarsbrook, 1965).
Although many of the above-mentioned processes have been studied under laboratory conditions or in simple field experiments, information on N-availability in intensive vegetable production in tropical, rice-based lowlands is rather limited. The aim of this study is, therefore: (1) to evaluate the impact of seasonal variations in soil moisture on soil N-availability, crop nutritional status, and yield, (2) to determine the relative importance of water stress and nitrogen availability on crop yields, and (3) to estimate potential leaching losses of N in intensive, year-round vegetable production on two different bed heights under two N-fertilization regimes.
2 Materials and methods
2.1 Site, systems, and crop management
From 1992 to 1995, the feasibility of year-round vegetable production on the same plot was studied at the experimental farm of AVRDC, Shanhua in southern Taiwan (23º N latitude). Monthly sums of precipitation are presented in Fig. 1. Soil at the experimental site was an alluvial sandy loam (0.5 % total N, 18 % clay containing illite and vermiculite, 27 % silt, 55 % sand). Cultivation systems consisted of traditional flat beds (20-25 cm high), which were prepared before sowing or transplanting each crop, and permanent high beds (50 cm high) constructed in spring 1992. Flat beds and high beds were 40 m long, divided into 20-m-long flat-bed plots and 4-m-long high-bed plots, randomized in a complete block design with four replications. During 1994/95 four vegetable crops, namely vegetable soybean (Glycine max. L. Merr; cv. ‘AGS 292’, AVRDC), Chinese cabbage (Brassica pekinensis Lour. Rupr.; cv. ‘ASVEG No. 1’, AVRDC), chili (Capsicum annuum L.; variety ‘Hot Beauty’, Known You Seed Co.), and carrot (Daucus carota L. ssp. sativus Hoffm. Arcang.; cv. ‘Parano’, Nunhems) were cultivated with two nitrogen fertilization rates: (1) the commonly used traditional (‘standard’) N application rate, and (2) a rate reduced by the amount of mineralized nitrogen before application (Nmin-method). Fertilizer nitrogen was either applied as ammonium sulfate and rototilled into the soil for all basal applications or applied to the soil surface for all side-dressings. Details of Nmin-contents and crop N fertilization are presented in Table 1. Vegetable crops were irrigated during dry periods with overhead water emitted from perforated pipes. Plant protection and other crop management practices followed standard AVRDC recommendations.
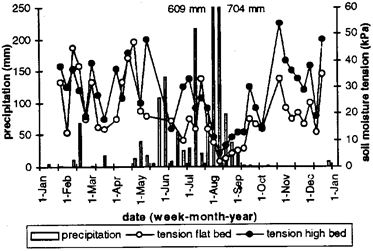
Fig. 1: Weekly precipitation and mean weekly soil moisture tension at 15-cm soil depth during 1994 in flat and high beds.
2.2 Measurement of soil moisture tension and calculation of water stress
Soil moisture tension was measured with vacuum gauge tensiometers installed at 15-cm soil depth within crop rows in one-half of a flat bed (one crop row), and one-half of two high beds (three crop rows each) with two replications. Readings were taken at approximately two-day intervals during the crop cultivation periods when field conditions allowed access. Total water stress was estimated for individual crops by a modified calculation of Taylor’s ‘mean integrated soil moisture tension’ (Taylor, 1952) to account for stress caused by overdry and overwet soil conditions according to the equation:
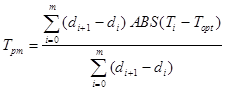
where: Tpm is the mean integrated soil moisture tension, i represents a single time, m represents the total number of tensiometer readings, d represents the Julian day of the year when a reading was made, (di+1-di) is the time interval in days between successive readings, Ti is the moisture tension at a single time, and Topt is an ‘optimum’ soil moisture tension, which was approximated for each crop by an iteration procedure.
2.3 Soil nitrogen analysis
Soil mineralized nitrogen was measured by sampling cropped soil 0 to 30-cm-deep and 30 to 60-cm-deep (three samples per plot) with a 2.0-cm-diameter punch tube at weekly intervals in four flat and four high beds (standard N application rate and Nmin-method; two replications). Between sampling and analyzing, samples were stored in a cooler. Extracted 1:2 by volume in 0.8 % KCl water solution, samples were filtered and analyzed for NO3 and NH4 using Merck’s RQflex reflectometer, Reflectoquant nitrate (5-225 ppm), and Reflectoquant ammonium (0.2-7.0 ppm) analytical test strips. The advantage of this analytical method is that several ions can be analyzed with ion-specific test-strips, that no calibration is needed, and that no further laboratory equipment is needed. Disadvantages are the limited concentration range and costs of the strips. Each batch of test strips was supplied with a bar-code which contained information for wave-length correction and a batch-specific calibration curve. The bar-code initialized the battery-powered, hand-held reflectometer. Each test strip had two reactive pads to produce a mean value. Before analysis, the meter’s clock was started at the same time as the strip was dipped into a sample. 5 seconds before the clock counted down a test-specific time (NO3: 60 seconds, NH4: 8 minutes), the strip was inserted into the meter and a concentration value displayed. With additional clocks, more strips could be analyzed when strips were read consecutively at twenty-second intervals. The meter was tested against a range of nitrate standard solutions with satisfactory results. Holden and Scholefield (1995) confirmed the reliability of the test.
2.4 Plant nitrogen analysis
Plant sap nitrate analysis (SN test) is recognized as a useful tool for determining nitrogen nutrition status, and hence crop yield (Olsen and Lyons, 1994). In this method, petioles of recently matured leaves are used for analysis (Prasad and Spiers, 1984). Petioles were collected from eight newly expanded leaves per plot for vegetable soybean and carrot. Twenty complete leaves per plot were sampled for chili, and five Chinese cabbage midribs per plot. Between sampling and analysis, petioles were stored on ice. Plant sap was expressed with a garlic press and diluted with deionized water to fit the range of the test strips for NO3-N analysis.
2.5 Nitrification of ammonium fertilizer
Ammonium sulfate was applied at a rate of 60 kg N/ha to flat bed and high bed plots (8 and 12 m2, three replications) kept free of crops and weeds on three different dates: 11 January, 23 March, and 13 June 1995. Both NH4-N and NO3-N were measured daily in samples taken from the 0 to 30-cm soil layer until ammonium concentrations were less than 1 ppm. Soil nitrogen content before fertilizer application was subtracted from measured concentrations.
2.6 Root length density measurement
Root length was measured in one flat bed and one high bed shortly before final crop harvest using the ‘gridline intersect method’ (Newman, 1966). Soil was sampled with a 2.0-cm-diameter punch tube to a depth of 60 cm in distances of 20 cm from the edge towards the centre of the beds with two replications. The soil column was cut into 10-cm-long sections and roots separated by carefully washing the soil through a fine (0.15 mm) sieve. The roots were spread out uniformly in a petri dish and put upon a grid of lines with an interline distance of 1.27 cm. Root length in centimeter was determined by the number of counted root/gridline intersects (Giovanetti and Mosse, 1980). Three readings were made for each sample by rearranging the roots in the petri dish. Root length density (cm/cm3) was calculated by dividing the mean of root length readings (cm) by the volume of the soil sample (cm3). Since too many roots of weedy species were found in the topmost 10-cm soil depth, those data were excluded.
2.7 Crop yield determination
Crop yields were recorded for bordered areas from individual rows. Soybean pods were hand-picked and fresh weight at one harvest and chili peppers were picked on 1st November, 15th November, 28th November, and 20th December. Chinese cabbage net (marketable) yield was determined as head yield without wrapper leaf. Carrot yield was recorded as root yield without leaf.
2.8 Multiple regression of yield on water stress and soil-N availability
Values of final mean integrated soil moisture tension, average soil-NO3 content and net yield in three plots were transformed to percentages of their joint mean for each of the four vegetables. The pooled data was analyzed with multiple regression of (relative) net yield on (relative) water stress and (relative) soil-NO3 content.
3 Results
3.1 Moisture tension and available nitrogen in the root zone
Seasonal differences in precipitation were reflected in soil water status with low weekly means of moisture tension during the peak rainy season particularly on flat beds (Fig. 1). Soil nitrate content followed the same trend (Fig. 2). The amount of ammonium was usually not higher than a few kilograms per hectare except immediately after application of ammonium fertilizer.
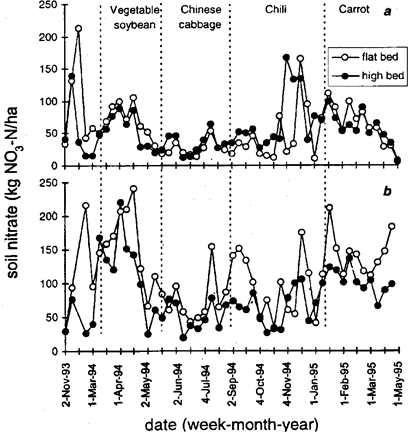
Fig. 2: Weekly soil contents of available NO3-nitrogen at 0 to 30-cm soil depth with 2 N-fertilizer application rates; (a) Nmin-method and (b) traditional (standard) rate in two cultivation systems: flat and high beds.
With only small amounts of fertilizer in the Nmin-treatment (Table 1), soil NO3 was highest during the dry season and lowest during the wet season, peaking at the end of the dry season late April and May 1994 and 1995 (Fig. 2 a). There was less NO3 in flat than in high beds in the wet season, but more during the dry season.
|
Table 1: Soil Nmin contents (mean ± standard error) and N-fertilizer application rates used for four vegetable crops cultivated in two cultivation systems during 1994/95 |
|||||||||||||||
|
Crop |
Vegetable soybean |
|
Chinese cabbage |
|
Chili |
|
Carrot |
||||||||
|
Cultivation period (week-month) Year |
1-Mar to 4-May 1994 |
|
4-May to 3-Jul 1994 |
|
4-Aug to 4-Dec 1994 |
|
2-Jan to 1-Apr 1995 |
||||||||
|
Date of application (week-month) |
1-Mar |
1-Apr |
1-May |
|
4-May |
2-Jun |
4-Jun |
|
3-Jul |
4-Aug |
2-Nov |
|
2-Jan |
4-Mar |
|
|
Nmin -content before fertilization |
|||||||||||||||
|
Flat bed (kg NO3-N/ha) |
43 ± 5.0 |
120 ± 10.2 |
51 ± 4.1 |
|
22 ± 2.8 |
32 ± 5.5 |
21 ± 1.2 |
|
16 ± 2.4 |
52 ± 6.0 |
23 ± 4.7 |
|
18 ± 2.8 |
52 ± 7.8 |
|
|
High bed (kg NO3-N/ha) |
16 ± 3.9 |
101 ± 7.2 |
20 ± 2.0 |
|
19 ± 1.6 |
39 ± 4.4 |
13 ± 2.0 |
|
20 ± 2.9 |
25 ± 2.5 |
21 ± 4.9 |
|
25 ± 3.5 |
48 ± 9.4 |
|
|
Fertilizer application rate |
|||||||||||||||
|
Traditional rate (kg N/ha) |
20 |
20 |
20 |
|
60 |
30 |
30 |
|
50 |
50 |
50 |
|
60 |
60 |
|
|
Nmin-method (kg N/ha) |
0 |
0 |
0 |
|
201 |
0 |
01 |
|
30 |
20 |
30 |
|
40 |
10 |
|
|
1 Nmin-calculation included expected N-release from incorporated vegetable soybean residues |
|||||||||||||||
When N-fertilizer was applied at the recommended (standard) rate (Fig. 2 b), soil NO3-contents were generally greater in flat beds irrespective of season.
3.2 Nitrification of ammonium fertilizer
Biological oxidation of ammonium to nitrate follows Michaelis-Menten reaction kinetics (Richter, 1987). Hyperbolic-type decreases in ammonium and increases in soil nitrate were approximated with quadratic regressions in Fig. 3. In the dry season, ammonium was completely oxidized to nitrate in flat and high beds within 12 days after application (Fig. 3 a). Although irrigation water was applied at rates of 17, 9, and 25 mm on days 1, 5, and 9, soil moisture tension did not fall below 10 kPa throughout the experiment. Irrigation rates in the second experiment during the transition phase from dry to wet season (Fig. 3 b) were 38 mm on day 1 and 22 mm on day 5. Up to day 8, soil moisture tension was above 10 kPa, but fell below that after rainfall of 49 mm and 7 mm on days 8 and 10. Nitrification proceeded in a similar way as in the first experiment, but NO3-contents on flat beds decreased soon after the rainfall events. In the wet season, ammonium sulfate was applied to a completely saturated soil (tension < 5 kPa) (Fig. 3 c). Soil moisture tension increased steadily towards the end of the experiment after an initial rainfall of 65 mm on day 1. This time, ammonium could be detected in the soil for 3 weeks, indicating that nitrification was delayed.
3.3 Interrelationship of N-fertilization, soil N content, plant N concentration, and yield
The relationship between nutrient application and crop nutrient uptake and between nutrient uptake and crop yield can be presented in a three-quadrant diagram (van Keulen, 1982). In the present study this was modified to a four-quadrant diagram, to include the relationship between N-fertilizer application rate and soil nitrogen availability (indicated by the mean of soil NO3 content over the cropping period) in Figs 4 and 5. The average of plant sap nitrate concentration measured during cultivation substituted for total crop nutrient uptake.
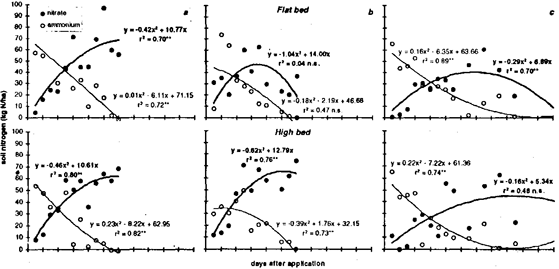
Fig. 3: Nitrification of ammonium-fertilizer (60 kg N/ha as (NH4)2SO4) applied on (a) 11 January, (b) 23 March, and (c) 13 June 1995 in two cultivation systems; (top) flat bed and (bottom) high bed as indicated by NH4-content and NO3-content in 0 to 30-cm soil depth. Lines represent quadratic trends for (thin line) NH4-N and (thick line) NO3-N.
There was no crop response to N-fertilization during the dry season when vegetable soybean and carrot were grown. This is indicated by flat slopes for both cultivation systems in quadrant d. During the wet season, N application resulted in higher soil nitrate contents on flat beds than on high beds (quadrant a). Slopes in quadrant b illustrate that particularly for the chili crop, these contents were not reflected in appreciably greater sap-NO3 concentrations in flat beds. Yields were substantially lower on those beds than on high beds (quadrant c and d).
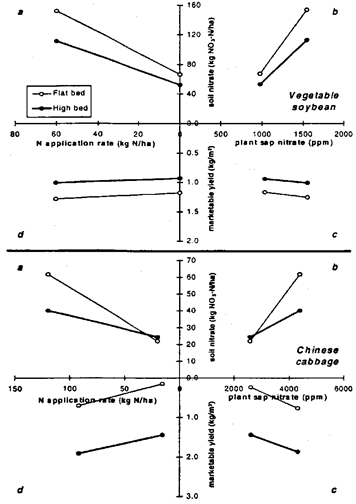
Fig. 4: Relationship of (a) N-fertilizer application and mean soil-nitrate content (0 to 30-cm soil depth), (b) mean soil-nitrate content and mean plant-sap-nitrate concentration, (c) mean plant-sap NO3 concentration and net yield, (d) net yield and N-fertilizer application of (top) vegetable soy-bean from March to May 1994 and (bottom) Chinese cabbage from May to July 1994 in flat and high beds.
3.4 Relative effect of moisture stress and nitrogen availability on vegetable yields
When mean integrated soil moisture tension (MISMT), averages of soil nitrate contents, and yields were converted to percentages of the mean of three measurements to allow comparison across crops (Table 2), the multiple regression analysis gave:
yield = 152.27 ** - 0.67 * · MISMT + 0.16 n.s. · soil NO3 (r2 = 0.40)
indicating that soil moisture stress was the more decisive of the two factors in limiting crop production.
|
Table 2: Transformation of measured data for mean integrated soil moisture tension (MISMT), mean soil NO3 content, and net yield to percentages of the mean of four vegetables in one flat-bed plot (FB) and two high-bed plots (HB1, HB2) for the multiple regression of net yield on water stress and soil N availability |
||||||||
|
|
Measured data |
|
Percentage of mean (%) |
|||||
|
|
FB |
HB1 |
HB2 |
Mean |
|
FB |
HB1 |
HB2 |
|
Vegetable soybean |
|
|
|
|
|
|
|
|
|
MISMT (kPa) |
18.15 |
34.64 |
31.87 |
28.22 |
|
64 |
123 |
113 |
|
Mean soil N-content (kg NO3-N/ha) |
80 |
45 |
72 |
66 |
|
121 |
68 |
109 |
|
Net yield (kg/m2) |
1.34 |
0.96 |
0.94 |
1.08 |
|
124 |
89 |
87 |
|
Chinese cabbage |
|
|
|
|
|
|
|
|
|
MISMT (kPa) |
4.50 |
4.48 |
8.08 |
5.69 |
|
79 |
79 |
142 |
|
Mean soil N-content (kg NO3-N/ha) |
24 |
21 |
21 |
22 |
|
109 |
22 |
96 |
|
Net yield (kg/m2) |
0.67 |
0.77 |
0.21 |
0.55 |
|
122 |
140 |
38 |
|
Chili |
|
|
|
|
|
|
|
|
|
MISMT (kPa) |
14.66 |
14.49 |
14.44 |
14.53 |
|
101 |
100 |
99 |
|
Mean soil N-content (kg NO3-N/ha) |
35 |
62 |
36 |
44 |
|
80 |
141 |
82 |
|
Net yield (kg/m2) |
0.22 |
0.41 |
0.30 |
0.31 |
|
71 |
132 |
97 |
|
Carrot |
|
|
|
|
|
|
|
|
|
MISMT (kPa) |
11.35 |
7.66 |
4.65 |
7.89 |
|
144 |
97 |
59 |
|
Mean soil N-content (kg NO3-N/ha) |
53 |
32 |
32 |
39 |
|
136 |
82 |
82 |
|
Net yield (kg/m2) |
3.09 |
2.65 |
3.10 |
2.95 |
|
105 |
90 |
105 |
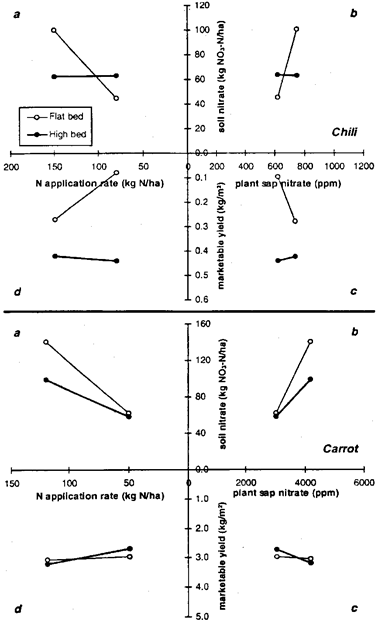
Fig. 5: Relationship of (a) N-fertilizer application and mean soil-nitrate content (0 to 30-cm soil depth), (b) mean soil-nitrate content and mean plant-sap-nitrate concentration, (c) mean plant-sap NO3 concentration and net yield, (d) net yield and N-fertilizer application of (top) chili from August to July 1994 and (bottom) carrot from January to April 1995 in flat and high beds.
3.5 Root length density and leaching of soil nitrate
Root density was restricted to the top 50-cm soil depth (Table 3). Root density in the whole profile was greater in high beds for all vegetables except carrot. Less roots were found above 20-cm depth, but root elongated more profusely in the 20 to 40-cm soil layer. Since root density was low beneath 40 cm, soil nitrate content at 30 to 60-cm soil depth indicated potential nitrogen loss through leaching. Nitrate contents were particularly high at the end of the dry season and low during the wet season (Fig. 6 a and b). Amounts of NO3 that were leached below the root zone were similar for high beds under both rates of N-fertilizer application, but potential leaching losses were higher in flat beds when the standard rate was applied.
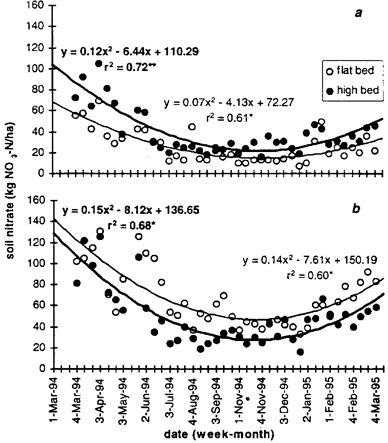
Fig. 6: Weekly soil contents of available NO3-nitrogen at 30 to 60-cm soil depth with 2 N-fertilizer application rates; (a) Nmin-method and (b) traditional (standard) rate; in AVRDC/Taiwan from 1994 to 1995 in flat and high beds. Lines represent quadratic trends for (thin line) flat beds and (thick line) high beds.
Table 3: Distribution of root length density (mean ± standard error; flat bed: n = 6, high bed: n = 14) of four vegetables cultivated on flat beds and high beds during 1994/95 |
||||||||||||
|
|
Vegetable soybean |
|
Chinese cabbage |
|
Chili |
|
Carrot |
|||||
|
Depth |
Flat bed |
High bed |
|
Flat bed |
High bed |
|
Flat bed |
High bed |
|
Flat bed |
High bed |
|
|
(cm) |
(cm/cm3) |
|
(cm/cm3) |
|
(cm/cm3) |
|
(cm/cm3) |
|||||
|
10-20 |
1.27 ± 0.066 |
1.05 ± 0.151 |
|
1.00 ± 0.205 |
0.81 ± 0.083 |
|
0.93 ± 0.352 |
0.91 ± 0.150 |
|
1.52 ± 0.259 |
1.27 ± 0.096 |
|
|
20-30 |
0.30 ± 0.027 |
0.83 ± 0.133 |
|
0.29 ± 0.055 |
0.61 ± 0.065 |
|
0.59 ± 0.250 |
0.79 ± 0.180 |
|
0.26 ± 0.057 |
0.42 ± 0.081 |
|
|
30-40 |
0.03 ± 0.017 |
0.34 ± 0.079 |
|
0.22 ± 0.089 |
0.46 ± 0.128 |
|
0.29 ± 0.122 |
0.50 ± 0.171 |
|
0.25 ± 0.168 |
0.44 ± 0.130 |
|
|
40-50 |
0.03 ± 0.012 |
0.21 ± 0.048 |
|
0.26 ± 0.078 |
0.20 ± 0.062 |
|
0.06 ± 0.019 |
0.14 ± 0.064 |
|
0.16 ± 0.015 |
0.27 ± 0.085 |
|
|
50-60 |
0.01 ± 0.006 |
0.02 ± 0.019 |
|
0.03 ± 0.039 |
0.01 ± 0.018 |
|
0.01 ± 0.004 |
0.02 ± 0.012 |
|
0.01 ± 0.008 |
0.02 ± 0.009 |
|
|
Mean |
0.33 |
0.49 |
|
0.36 |
0.42 |
|
0.38 |
0.47 |
|
0.44 |
0.48 |
|
4 Discussion
Although only small amounts of fertilizer-N were applied in the Nmin-treatment, soil nitrate accumulated during the dry season (Fig. 2 a). Nitrate accumulation in the dry season was observed in several seasonal wet-dry climates in the tropics by Greenland (1958). Although soil moisture is probably too low for maximal N mineralization, leaching of NO3 is minimal in this season (Reynolds-Vargas et al., 1994). Nitrate can accumulate in the surface soil through upward movement from subsoils when evaporation exceeds precipitation. Mineralization might have also been accelerated during the dry season through alternate drying and rewetting of the soil during irrigation cycles (McLaren and Peterson, 1965). Although nitrification of ammonium proceeded rapidly and completely in the dry season (Fig. 3 a), soil nitrate accumulated to levels that could not be explained by lack of leaching alone, since significant mineralization of N from the low soil organic matter pool could not be expected. Although not analyzed in this study, release and nitrification of nonexchangeable, clay-fixed ammonium may play a significant role in this process. Although it was shown that pools of mineralized, exchangeable and chemically fixed soil ammonium are in equilibrium (Drury and Beauchamp, 1991), considerable amounts of nitrogen can be present in this form (Hinman, 1964). This nitrogen may play a significant role in N nutrition of crops (Mengel and Scherer, 1981; Keerthisinge, 1984).
Soil nitrate contents peaked just before the onset of rainfall in the wet season (Fig. 2 and 8). This has consequences for crop production in this environment. Mineralization of soil nitrogen in the dry season can partially meet nitrogen requirements of vegetable crops so that additional N-fertilizer applications could be reduced. This finding can also explain the sometimes low recovery of fertilizer N in this season (AVRDC, 1995). This nitrogen quickly declined at the onset of rainfall in the wet season. The relative importance of denitrification and leaching during transition from dry to rainy season was not traced in this study, but both processes are known to harm the environment (AVRDC, 1995). The potential loss of soil nitrate is greatest under the cropping pattern winter vegetables-spring rice crop which is common in lowland Taiwan (Chiu, 1987) and other similar climates. This cropping system virtually eliminates percolation of nitrate to the groundwater (Terry and Tate, 1980), but accelerates denitrification of NO3. Buresh et al. (1993) described the important role of green manure between two rice cultivations in immobilizing mineralized NO3 to resist leaching, and cycling this N back to the soil N-pool so that it can be used by rice again. In highly intensive vegetable production, it is recommended to incorporate a vegetable with high N-absorption capacity (e.g., sweet corn) as a cropping component to remove high soil nitrate contents before the onset of the rainy season.
High fertilizer application rates on flat beds were reflected in contents of soil inorganic nitrogen, but not in plant sap NO3 concentrations in the rainy season. Crop roots were mainly composed of taproots and the root density was small, hence resulting in substantially lower yields. It could be concluded that high soil moisture, i.e., low water tension, accelerated taproot development with less branches and root hairs, and consequently reduced rootmass on flat beds, so that available soil nitrogen could not be effectively absorbed by crops. This brought about poor biomass production in vegetables and hence low yields. The nitrogen that was not absorbed was easily leached out of the root zone.
Wesseling (1974) stated that the efficiency of applied N-fertilizer depends largely on drainage conditions. On high beds where root systems of vegetables were much different from flat beds, available soil nitrogen was efficiently absorbed by vegetables and its productivity was kept high throughout the season (Kleinhenz et al., 1995). Roots were rich in long, slender and soft mainroots with a lot of branches and roothairs, thus resulting in huge rootmass. Therefore, the higher application rate of fertilizer-N did not result in greater potential leaching losses (Fig. 6). Overall, the direct impacts of excessive soil moisture in the rainy season and deficient soil moisture in the dry season were apparently more detrimental to vegetable growth than was limited availability of soil nitrogen. Similar findings for grain corn (Isfan, 1984) indicate that nitrogen effects were only secondary when soil water stress occurred.
5 Acknowledgments
We thank the German Federal Ministry of Economic Cooperation (BMZ) for funding and the Asian Vegetable Research and Development Center for its support in the implementation of this project.
6 Summary
Zusammenfassung
Der Einfluss saisonaler Variation von Bodenwassergehalt auf verfügbarem Bodenstickstoff, Stickstoffaufnahme und Ertrag wurde in einer ganzjährigen Kulturfolge von vier Gemüsearten von 1994 bis 1995 beim „Asian Vegetable Research and Development Center” (AVRDC) im tropischen Flachland von Taiwan untersucht.
Linderung von Wasserstress durch Hochbeete war eine Grundvoraussetzung für große Wurzelmasse und effiziente Absorbierung mineralisierten Bodenstickstoffs in der Regenzeit. Auf staunassen Flachbeeten war N-Absorbierung ineffektiv, Erträge blieben gering und mehr Nitrat wurde unter die auf die oberste Bodenschicht konzentrierte Wurzelzone ausgewaschen.
Während der Trockenzeit sammelte sich Bodennitrat an. Der Humusgehalt des untersuchten Reisbodens war gering und auch die geringere Auswaschungsrate konnte diese Akkumulierung nicht erklären. Freisetzung von an Tonmineralien gebundenem, nicht austauschbarem Stickstoff könnte eine wichtige Rolle gespielt haben. Stickstoffdüngemengen können während der Trockenzeit eingespart werden und es sollten Gemüsearten mit hohem Stickstoffanspruch vor Beginn der Regenzeit angebaut werden, um dem Verlust an Bodennitrat durch Auswaschung und Denitrifizierung vorzubeugen. Insgesamt war Wasserstress dem Gemüsewachstum mehr abträglich als Verfügbarkeit von Bodenstickstoff.
Efectos estacionales de la humedad del suelo para la disponibilidad de nitrógeno en el suelo, el contenido de N en la planta y el rendimiento de vegetales en un sistema tropical arrocero
Resumen
En un experimento de rotación de cultivos por todo el año con cuadro especies de vegetales en la Ilanura tropical de Taiwan (Asian Vegetable Research and Development Centre - AVRDC) se investigó durante 1994 y 1995 la influencia de la variación estacional del contenido de agua en el suelo al nitrógeno disponible en el suelo, a su absorción por las plantas y al rendimiento de los cultivos.
La disminución de estres acuático mediante de bancales elevados fue una suposición fundamental para un alto contenido de raices y una absorción eficiente del nitrógeno, mineralizado en el suelo, durante la época Iluviosa. En los bancales normales la absorción de N no fue eficiente, los rendimientos permanecieron en un nivel bajo y mas nitrato fue traslado a la capa por de bajo de las raices.
Durante la época de sequia el nitrato se acumuló en el suelo. El contenido de humus en el suelo arrocero investigado fue bajo y el traslado menor de nitrato durante la sequia no puede explicar la acumulación del mismo. La liberación de nitrógeno incambiable asimilado por los minerales arcillosos pudiera jugado un papel importante. Durante la sequia se puede eliminar la fertilización con nitrógeno. Antes de la época Iluviosa se debe cultivar especies de vegetates con alta exigencia al nitrógeno para evitar perdidas de nitrógeno acumulado en el suelo por traslado y denitrificación. En total el estrés acuático redujo mas ei crecimiento de los vegetates que la disponibilidad de nitrógeno.
7 References
1. Allison, F. E.; Kefauver, M.; Roller, E. M., 1953: Ammonium fixation in soils. Soil Science Society of America Proceedings, 18, 107-110.
2. AVRDC, 1995: AVRDC 1994 Progress Report. Asian Vegetable Research and Development Center, Shanhua, Tainan, Taiwan.
3. Buresh, R. J.; Chua, T. T.; Castillo, E. G.; Liboon, S. P.; Garrity D. P., 1993: Fallow and Sesbania effects on soil nitrogen dynamics in lowland rice-based cropping systems. Agronomy Journal, 85, 316-321.
4. Chiu, C. C., 1987: Evolution of farming systems in Taiwan. ASPAC Extension Bulletin 265. Food and Fertilizer Technology Center, Taipei, Taiwan.
5. Drury, C. F.; Beauchamp, E. G., 1991: Ammonium fixation, release, nitrification, and immobilization in high- and low-fixing soils. Soil Science Society of America Journal, 55, 125-129.
6. Giovanetti, M.; Mosse, B., 1980: An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist, 84, 489-500.
7. Greenland, D. J., 1958: Nitrate fluctuations in tropical soils. Journal of Agricultural Science, 50, 82-92.
8. Harmsen, G. W.; Kolenbrander, G. J., 1965: Soil inorganic nitrogen. In: Soil Nitrogen. Eds. W. V. Bartholomew and F. E. Clark. American Society of Agronomy, Madison, Wisconsin, 43-71.
9. Hinman, W. C., 1964: Fixed ammonium in some Saskatchewan soils. Canadian Journal of Soil Science, 44, 151-157.
10. Holden, N. M.; Scholefield, D., 1995: Paper test-strips for rapid determination of nitrate tracer. Communications in Soil Science and Plant Analysis, 26, 1885-1894.
11. Isfan, D., 1984: Corn yield variation as related to soil water fluctuation and nitrogen N-fertilizer. II. Soil water-nitrogen-yield relationships. Communications in Soil Science and Plant Analysis, 15, 1163-1174.
12. Ishii, K., 1986: Soil management for paddy-upland rotation. In: Paddy Field Diversion and Upland Crop Production. Eds. S. C. Hsieh and D. J. Liu. Taichung District Agricultural Improvement Station, Taichung, Taiwan, 139-152.
13. Justice, J. K.; Smith, R. L. 1962: Nitrification of ammonium sulfate in a calcareous soil as influenced by combinations of moisture, temperature, and levels of added nitrogen. Soil Science Society of America Proceedings, 26, 246-250.
14. Keerthisinghe, G.; Mengel, K.; DeDatta, S. K., 1984: The release of nonexchangeable ammonium (15N labelled) in wetland rice soils. Soil Science Society of America Journal, 48, 291-294.
15. van Keulen, H., 1982: Graphical analysis of annual crop response to fertiliser application. Agricultural Systems, 9, 113-126.
16. Kleinhenz, V.; Schnitzler, W. H.; Midmore, D. J., 1995: High bed systems for off-season vegetable production in the Tropics and Subtropics. Entwicklung und Ländlicher Raum, 4, 26-28.
17. Koch, E., 1987: Bodenuntersuchung. VDSF Verlags- und Vertriebs-GmbH, Offenbach am Main.
18. McLaren, A. D.; Peterson, G. H., 1965: Physical chemistry and biological chemistry of clay mineral-organic nitrogen complexes. In: Soil Nitrogen. Eds. W. V. Bartholomew and F. E. Clark. American Society of Agronomy, Madison, Wisconsin, 261-286.
19. Mengel, K.; Scherer, H. W., 1981: Release of nonexchangeable (fixed) soil ammonium under field conditions during the growing season. Soil Science, 131, 226-232.
20. Miller, R. D.; Johnson, D. D., 1964: The effect of soil moisture tension on carbon dioxide evolution, nitrification, and nitrogen mineralization. Soil Science Society of America. Proceedings, 28, 644-647.
21. Newman, E. I., 1966: A method of estimating the total length of root in a sample. Journal of Applied Ecology, 3, 139-145.
22. Nõmmik, H., 1965: Ammonium fixation and other reactions involving a nonenzymatic immobilization of mineral nitrogen in soil. In: Soil Nitrogen. Eds. W. V. Bartholomew and F. E. Clark. American Society of Agronomy, Madison, Wisconsin, 200-260.
23. Olsen, J. K.; Lyons, D. J., 1994: Petiole sap nitrate is better than total nitrogen in dried leaf for indicating nitrogen status and yield responsiveness of capsicum in subtropical Australia. Australian Journal of Experimental Agriculture, 34, 835-843.
24. Patrick, W. H.; Wyatt, R., 1964: Soil nitrogen loss as a result of alternate submergence and drying. Soil Science Society of America Proceedings, 28, 647-653.
25. Prasad, M.; Spiers, T. M., 1984: Evaluation of a rapid method for plant sap nitrate analysis. Communications in Soil Science and Plant Analysis, 15, 673-679.
26. Reynolds-Vargas, J. S.; Richter, D. D.; Bornemisza, E., 1994: Environmental impacts of nitrification and nitrate adsorption in fertilized andisols in the Valle Central of Costa Rica. Soil Science, 157, 289-299.
27. Richter, J., 1987: The Soil as a Reactor. Catena Verlag, Cremlingen.
28. Scarsbrook, C. E., 1965: Nitrogen availability. In: Soil Nitrogen. Eds. W. V. Bartholomew and F. E. Clark. American Society of Agronomy, Madison, Wisconsin, 486-501.
29. Sowden, F. J., 1976: Transformations of nitrogen added as ammonium and manure to soil with a high ammonium-fixing capacity under laboratory conditions. Canadian Journal of Soil Science, 56, 319-331.
30. Stanford, G.; Epstein, E., 1974: Nitrogen mineralization - water relations in soils. Soil Science Society of America Proceedings, 38, 103-107.
31. Taylor, S. A., 1952: Estimating the integrated soil moisture tension in the root zone of growing crops. Soil Science, 73, 331-339.
32. Terry, R. E.; Tate, R. L., 1980: Effect of flooding on microbial activities in organic soils: nitrogen transformations. Soil Science, 129, 88-91.
33. Wesseling, J., 1974: Crop growth and wet soils. In: Drainage for Agriculture. Ed. J. van Schilfgaarde. American Society of Agronomy, Madison, Wisconsin, 7-38.