

Physiological studies on edible bamboo
Midmore, D.J.; Kleinhenz, V., 2000
Access to Asian Foods, 6, 6-8
Physiological Studies on Edible Bamboo
David J. Midmore & Volker Kleinhenz
Interest amongst the Australian commercial farming community in the cultivation of bamboo is high, and spreading across the States and Territories (see Issues 2 and 5 of this Newsletter). Since 1994 the Plant Sciences Group of the Primary Industries Research Centre at Central Queensland University has been researching issues of production and post-harvest pertinent to the needs of producers of bamboo shoots. In the main, this has related to studies on the timing and range of harvest periods across species and locations (important for a co-ordinated approach to market supply), on requirements for water and mineral nutrients, on clump management (culling of culms, and canopy management), and on packaging, transport and storage protocols to maintain shoots in a fresh, acceptable condition to the consumer. Herein, we report briefly on the nutrient and irrigation studies and on some of the results from post-harvest research.

Near complete canopy of Bambusa oldhamii in
South East Queensland
Nutrition
Commercial producers of bamboo for shoots and timber, and those growing bamboo for waste water dissipation, are seeking figures to help estimate best nutrient and irrigation requirements.
It is our belief that analyses of plant tissue are a better indicator of nutrient status of bamboo than are soil analyses. Our results starting in 1997 with the first fertilisation trial with bamboo conducted in Australia point to the great demand by bamboo for nutrients, especially for nitrogen. In order for producers not to depend on recommendations for fertilisation of bamboo based on application rates from isolated field experiments or worse, from unratified overseas information (eg growers’ handbooks) we suggest that farmers base their fertilisation (organic or inorganic) on results of plant analyses in their own crop. For this, the last fully expanded leaf should be used since this plant part is usually the best indicator of the current nutrient status of the plant. Response of crops to available nutrients in their growth media usually follows a hyperbolic relationship with great yield response to nutrient application at low plant nutrient levels and a decreasing rate of response with increasing plant nutrient contents (the ‘Michaelis-Menten’ relationship). We follow the procedures of the ‘Diagnosis Recommendation Integrated System’ (DRIS) which is the basis for fertilisation in many important plantation crops such as oil palm, coffee and cocoa but also annual crops such as vegetables. For those crops, relationships and cross-relationships between macronutrients and even micronutrients have been studied to guide fertilisation. An example is presented in Figure 1. Out data suggest that a “lower limit’ for leaf N is C. 2.0%N, and above 3.5%N nitrogen supply is excessive and results in luxury uptake, most likely serving no good to the plant.
Although we do not have sufficient data to show the effect of plant age on affinity for N, the calculated hyperbola (Figure 1) may be used as a guide as to how much N fertiliser needs to be applied to raise N concentrations in bamboo leaves. The data points at 0 kg ha-1 N application represent the natural ‘fertility’, ie N mineralisation rate of the particular soil. The small differences in leaf-N values at 0 kg ha-1 N can be explained by differences in soil ‘fertility’ at two sites. The regression line indicates that relatively little N is required to lift leaf-N from low levels but more from higher levels. The equation estimates the theoretical maximum leaf-N concentration at 4.13 percent. Our suggested ‘optimum’ of 3.0 percent leaf N is at a level below which bamboo has a great affinity for N and above which plants respond only slightly to additional units of N application.
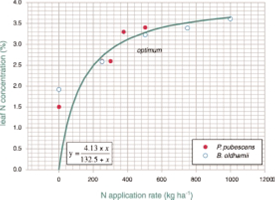
Figure 1. Relationship between N application rate and leaf N
concentration in two bamboo species
The following example resulting from our research with Bambusa oldhamii may demonstrate a practical application of the suggested fertilisation strategy for farmers. When leaf N in unfertilised plants was 1.8 percent, (360 – 120=) 240 kg ha-1 N was needed to raise leaf N to 3.0 percent. If leaf N would have dropped to 2.4 percent 4 – 6 months after that application of 240 kg ha-1, (360 – 180 =) 180 kg ha-1 N would have been required to raise leaf N back to 3.0 percent. From this, the total annual N application rate for 2 – 3 applications per year after the first year would sum up to (2 to 3 x 180 =) 360 to 540 kg ha-1year-1N. This range confirms our first estimates for optimum fertiliser application in 1997. We are now testing this DRIS application for different leaf-N levels (Control, 2.5, 3.0 and 3.5% N) in two bamboo species (B. oldhamii and Dendrocalamus latiflorus) in three sites.
Water use
Our preliminary studies of transpiration (the loss of water through evaporation from the leaf surface) and photosynthesis (the fixation of carbon from carbon dioxide, into carbohydrates variously used within the plant) suggest that the older the culm, the greater the rate of transpiration, but the lower the rate of photosynthesis. For efficient use of water, younger culms (2 years or less) are indicated; for waste water dissipation a greater proportion of older culms within a clump, maybe more suitable.
Based upon our data on rates of transpiration (ie, the volume of water lost per unit area of leaf surface per unit time), and on the size of the canopy (the leaf bulk) of a mature plantation, we estimate that bamboo can use up to 3,300 mm per year under well-watered conditions. Current experiments are refining this estimation. This figure does not imply that for optimal shoot-timber yields such quantity should be available, rather it indicates the maximal dissipation rate of a solid stand of bamboo.

Preparing bamboo shoot for storage trial
Post-harvest
Three fundamental processes of quality loss in fresh bamboo shoots after harvest comprise:
1. weight loss through transpiration,
2. weight loss through respiration and
3. discolouration and fungal infection.
Weight loss, discolouration and fungal infection can be reduced through appropriate packaging and cooling. Generally, there are two parameters of ‘cooling’ that affect shelf-life of horticultural commodities: the optimum storage temperature and how quickly the temperature of the produce is reduced to that storage temperature.
Fresh bamboo shoots in Asia are traditionally marketed at ambient temperatures and unpackaged. When temperature cannot be controlled, shelf-life of shoots is shortened by transpirational weight loss, and is no longer than one day if measures for increasing humidity around the shoot are not enforced.
High rates of respiration (ie, the loss of storage products such as sugars to maintain cellular integrity, resulting in production of CO2) and transpiration lead to rapid weight loss and loss of quality in shoots. Packaging in low-density polyethylene film significantly reduces weight loss (even at 20° C shelf-life could reach 6 days), and in combination with storage at 2° C which reduces respiration and possible fermentation, could extend shelf-life to one month in the absence of discolouration and fungal infection.
Hydro-cooling is much more effective than air cooling in reducing the internal temperature of shoots below that at which significant respiration occurs. Larger shoots require greater time for cooling (Figure 2); early morning harvests would require less cooling than would shoots harvested during the day time.
A more comprehensive report on the outcomes of this research, which terminates in mid-2000, will be published later this year by RIRDC.
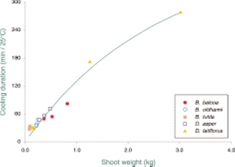
Figure 2. Duration of hydro-cooling time to reduce
internal temperature of bamboo shoots by 25°C

Dr Kleinhenz
Plant Sciences Group
Primary Industries Research Centre
Central Queensland University
ROCKHAMPTON QLD 4702
Tel: (07) 4930 9770
Fax: (07) 4930 9255
Email: d.midmore@cqu.edu.au
Email: v.kleinhenz@cqu.edu.au
website: http://science.cqu.edu.au/psg/